www.acsprf.org
Reports: DNI349914-DNI3: Renewable Energy Catalysis: The Study of Water Oxidation by Binuclear Metal Complexes
Liviu M. Mirica, PhD , Washington University
For the past 24 months we have been successful in synthesizing ligands based on two of our bridging linker frameworks, survey their coordination chemistry to generate Ni, Cu, and Co complexes, and investigate the electrochemistry and water oxidation reactivity of the corresponding bis(m-hydroxo) dinuclear complexes. We are currently extending our studies to other transition metal ions that can access high oxidation states and can be employed in various small molecule activation processes.
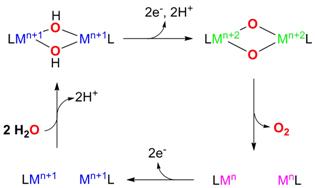
Figure 1. Targeted electrocatalytic water-oxidation cycle using a binuclear metal complex.
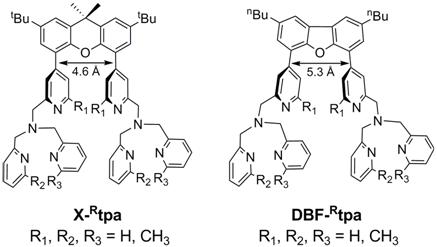
While several reaction schemes have been considered for water-oxidation model systems, the formation of O2 through the reductive elimination of a bis(m-oxo) binuclear complex, to the best of our knowledge, has not been employed before for water-oxidation catalysis (Figure 1). Specifically, binuclear metal complexes with water-derived hydroxide bridging ligands will be oxidized electrochemically to generate the bis(m-oxo) dinuclear metal species, which will undergo reductive elimination to generate O2. The targeted ligands are binucleating variants of the tetradentate ligand, tris[(2-pyridyl)methyl]amine (tpa); use of different rigid linkers will allow the control of the metal-metal distance by modulating the spatial distribution of the two metal centers. We were successful in synthesizing the binucleating ligand xanthene-bis(tris(2-pyridylmethyl)amine), X-tpa, as well as the dibenzofuran-bridged equivalent DBF-tpa in 30% to 50% yields for a six-step synthesis (Figure 2). For each ligand framework we have synthesized six ligand variants with one, two, and three methyl groups in the 6-pyridyl position (R1, R2, R3 = H or CH3, Figure 2), which will allow us to further control the reactivity of the targeted metal/O2 intermediates.
Figure 2. Proposed ligands for dinuclear metal complexes (the boxed ligands were successfully synthesized).
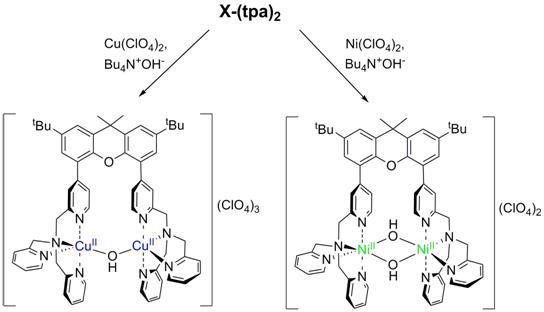
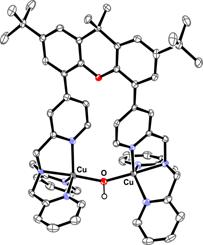
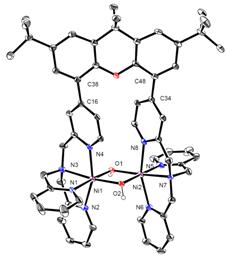
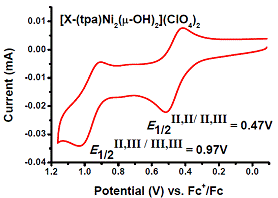
The ability of the synthesized ligand X-tpa to form dinuclear complexes was tested initially in reactions with Cu and Ni salts. Interestingly, when Bu4NOH was added to X-tpa and Cu(ClO4)2, a (m-hydroxo)dicopper(II) complex was formed (Figure 3). By contrast, the use of Ni(ClO4)2 generated a bis(m-hydroxo)dinickel(II) complex (Figure 3). Cyclic voltammetry (CV) experiments reveal the oxidation of the [(X-tpa)Cu2(OH)](ClO4)3 complex occurs at quite high potential (~1.2 V vs. Fc/Fc+), while the [(X-tpa)Ni2(OH)2](ClO4)2 complex shows two oxidation waves at 0.47 V and 0.97 V vs. Fc/Fc+ that have been assigned to the Ni2(II,II)/(II,III) and Ni2(II,III)/(III,III) redox couples, respectively (Figure 4). These results suggest that the presence of two hydroxide bridging ligands is needed for an accessible oxidation potential.
Figure 3. Synthesis and crystal structures of dinuclear Cu and Ni hydroxide complexes with the X-tpa ligand.
Figure 4. Cyclic voltammetry of [(X-tpa)Ni2(OH)2](ClO4)2 in MeCN (100 mV/s scan rate).
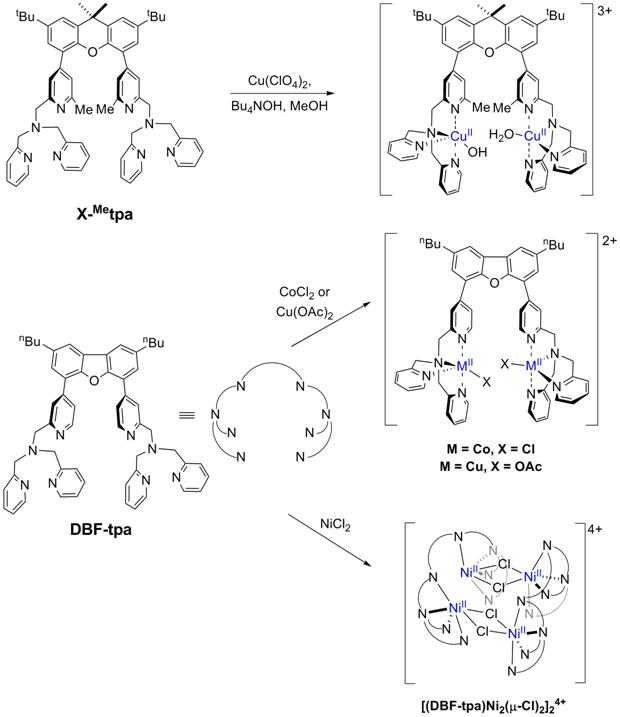
For the X-Metpa ligand (R1 = Me, Figure 2), the steric hindrance of the methyl groups led to formation of a dinuclear Cu complex that has no bridging ligands between the two Cu centers (Figure 5, top). Moreover, no (X-Metpa)Ni complex could be isolated under several reaction conditions. The second type of binucleating ligand, DBF-tpa, was designed in order to enforce a slightly longer distance between the two metal centers (Figure 2). While various metal salts were employed in the synthesis of the targeted hydroxide-bridged dinuclear complexes, no such complexes were obtained; instead complexes with non-interacting metal centers were isolated (Figure 5, middle). In one case, use of NiCl2 led formation of a dimer-of-dimer tetranuclear complex with chloride ligands that bridge intermolecularly the Ni centers (Figure 5, bottom). While this geometry was not anticipated, it can be envisioned as appropriate for the targeted water oxidation mechanism. Unfortunately, many attempts to replace the chloride ligands with hydroxide led to decomposition of the complex. Based on these results, we consider that the use of the dibenzofuran linker projects the metal centers too far apart from each other and thus is not an appropriate rigid linker for our targeted water oxidation states.
Figure 5. Synthesis and crystal structures of dinuclear Cu, Co, and Ni hydroxide complexes with the X-(tpa)2 and DBF-tpa ligand.
Overall, these initial results confirm our assumption that bis(m-hydroxo) bimetallic complexes should have accessible oxidation potentials and under the appropriate conditions should allow the formation of high-valent complexes. In the case of Ni complexes, it seems that the formation of a Ni(III)Ni(III) intermediate can be achieved, which will be essential for being able to effect water oxidation. Interestingly, preliminary results show that the [(X-tpa)Ni2(OH)2](ClO4)2 in presence of Ce(IV) lead to formation of ~50% of O2. We are currently investigating the electrochemical oxidation of various Ni complexes in an attempt to oxidize water catalytically. However, the use of tpa-derived ligands seem to leads to leaching of Ni2+ ions in solution and likely formation of a heterogeneous catalyst at the anode that catalyzes water oxidation, similar to the reports of Nocera et al. In order to develop a molecular catalyst that will allow us to investigate the mechanistic details of water oxidation and O-O bond formation, we plan to employ macrocyclic tetradentate ligands in the synthesis of dinuclear complexes (see below).
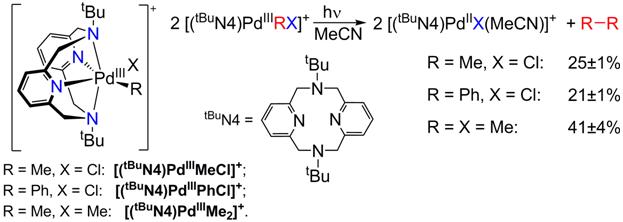
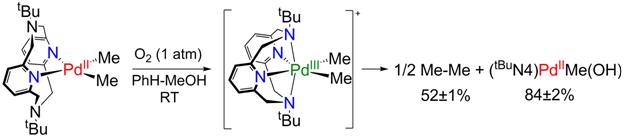
While designing various ligands that can stabilize transition metal ions in higher oxidation states, we have found that the tetradentate ligand N,N'-ditertbutyl-2,11-diaza[3.3](2,6)pyridinophane, tBuN4, is exhibiting an interesting reactivity behavior. As part of this work, we have recently reported (J. Am. Chem. Soc. 2010, 132, 7303-7305) that tBuN4 stabilizes Pd(III) centers and allows for the isolation and characterization of the first mononuclear organometallic Pd(III) complexes. These complexes undergo a light-induced elimination of the homocoupled products ethane or biphenyl, the observed formation of ethane from monomethyl Pd complexes being unprecedented (Figure 6). We later found that (tBuN4)PdIIMe2 undergoes facile oxidation in presence of O2 or peroxides (H2O2 or tBuOOH) to form the Pd(III) species [(tBuN4)PdIIIMe2]+, followed by formation of ethane and the monomethyl complex (tBuN4)PdIIMe(OH) (Figure 7). Moreover, the latter product can further react and activate the weakly acidic C-H bonds of organic substrates such as acetone or terminal alkynes (J. Am. Chem. Soc., accepted). Overall, these results point to a possible catalytic cycle that can be employed for the oxidative coupling of C-H bonds.
Figure 6. Stable mononuclear PdIII complexes and their light-induced reactivity.
Figure 7. Oxygen-induced ethane elimination from the (tBuN4)PdIIMe2 complex.