www.acsprf.org
Reports: ND1048730-ND10: Engineering the Photosensitizer-Semiconductor Interface in Dye-Sensitized Solar Cells
Michael R. Detty, PhD , State University of New York at Buffalo
World energy demand is predicted to double by 2050 and triple by 2100. In the long term, solar energy is the most practical and abundant alternative energy source. The amount of solar energy reaching the earth's surface per hour (4.3 x 1020 J) exceeds the current annual world energy consumption (4.1 x 1020 J). However, solar energy currently provides just 0.1% of the world's electricity supply. Efficient solar energy conversion requires absorption of solar illumination followed by reduction and oxidation processes which compete with charge recombination. The light-to-electrical energy conversion mechanism of dye-sensitized solar cells (DSSCs) using nanocrystalline TiO2 involves electron injection by the photoexcited sensitizer followed by electron transport through the TiO2 film and the external circuit. The oxidized sensitizer is reduced by an electron donor in the electrolyte, which is subsequently re-reduced at the counter electrode. In addition, charge separation at the semiconductor-photosensitizer interface leads to long-lived charge-separated states, enabling productive redox processes to compete with electron-hole recombination.
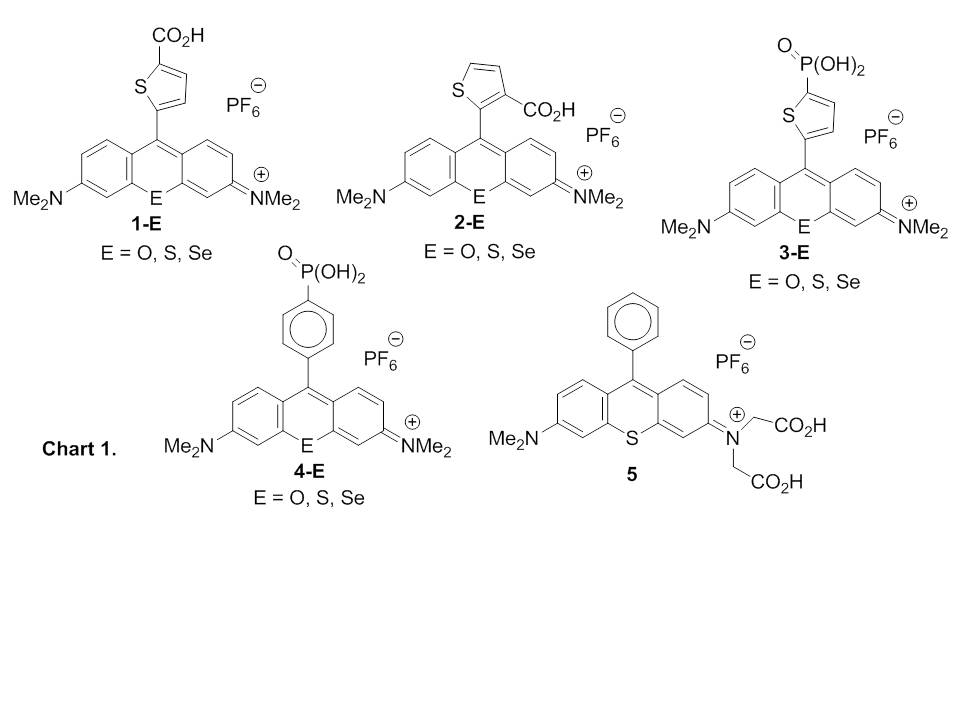
We have been examining chalcogenorhodamine dyes that form aggregates on nanocrystalline TiO2. These aggregated organic photosensitizers broaden the absorbance bands to both the shorter-wavelength side (H-aggregates) and the longer-wavelength side (J-aggregates) of the unaggregated dye absorbance maximum. Our initial studies indicated that incident-photon-to-current efficiency (IPCE) in several DSSCs based on H-aggregated dyes 1-E (E = O, S, Se) was much higher than in DSSCs based on amorphous, non-aggregated dyes 2-E (E = O< S, Se). The carboxylic acid functionality in dyes 1-E and 2-E is necessary to bind the dyes to the TiO2 surface.
Unfortunately, a single carboxylic acid group did not give appropriate binding affinity for the titania to produce long-lived devices and to allow photostability studies on TiO2 and ZrO2. We successfully prepared the phosphonic acid analogues 3-E and 4-E as well as the dicarboxylic acid analogue 5. All of these compounds had binding affinities that were 103to 104 times higher than the corresponding monocarboxylic acids. Furthermore, the phosphonic acid derivatives 3-E and the carboxylic acid derivatives 1-E had values of IPCE that were essentially identical over the solar spectrum. Analogues 3-O and 3-Se had integrated values of IPCE that were greater than those observed for the N3 ruthenium-based dye.
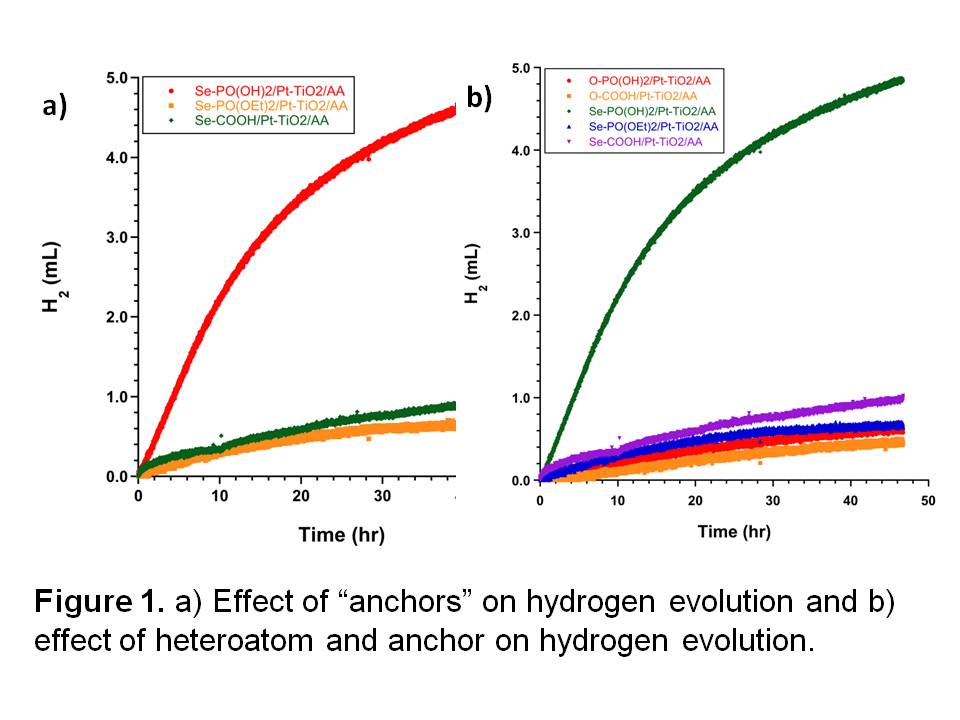
Our latest studies with 3-Se suggest that it is the most efficient photosensitizers ever examined for the evolution of hydrogen from water in a heterogeneous system with platinized titania. The following work was done in collaboration with Prof. Rich Eisenberg and Dr. William Eckenhoff at the University of Rochester. In the presence of ascorbic acid (AA) as a sacrificial electron donor and platinized titania, dye 3-Se was still producing hydrogen after 48 h of irradiation gave nearly and gave > 11,500 turnovers for the production of hydrogen in 24 h compared to 900 turnovers for dye 1-Se, the with a carboxylic acid anchor. The production of hydrogen tracks the triplet yield [as measured by the singlet oxygen yield (Q)] as shown by the much greater production of hydrogen from 3-Se relative to 3-O, which suggests that electron transfer is more competitive from the longer-lived triplet state..
In future studies, we shall prepare other chalcogenorhodamine derivatives with phosphonic acid or dicarboxylic acid functionality on the amino substituents in order to examine parameters for H- or J-aggregation. We shall also examine the oxidized photosensitizer on TiO2 by transient techniques in order to examine lifetimes and recombination rates. We shall also examine ways to optimize the evolution of hydrogen both in heterogeneous and homogeneous systems. In particular, we shall examine the lifetimes of the reduced photosensitizer as a means to prolong the period of hydrogen evolution.
The PRF support has allowed the PI to become involved with solar fuels and solar electricity by providing support for a core group of students. The graduate students on this project have benefited not only from the financial support, but also from the breadth of science involved in this project: synthesis, catalysis, photophysics, materials science. One undergraduate student participating in this project has been accepted for graduate studies in chemistry at a top-twenty school in chemistry.