www.acsprf.org
Reports: ND1049207-ND10: High-Pressure Kinetic Studies of Formation, Phase Transition and Crystal Growth of Methane Hydrates in Dynamic-DAC
Choong-Shik Yoo , Washington State University
Natural gas hydrates are the most abundant in nature and the largest energy resource of all fossil fuels, made of hydrogen-bonded cages of water molecules, containing a wide range of guest molecules. This project investigates the chemical mechanism and kinetics of methane hydrate formation, decomposition and phase transitions, using novel dynamic-diamond anvil cells (d-DAC), Raman spectroscopy, and optical microscopy.
Second year progress includes:
· Discovery of high-density amorphous ice at ambient temperature - well above crystallization temperature in “no-man's land”.
· High-pressure kinetic studies on formation and phase transitions of methane hydrates, which show strong compression dependences.
· High-pressure kinetic studies of water solidification in dynamic-DAC (complement results above).
A. Discovery of high-density amorphous ice at ambient temperature:
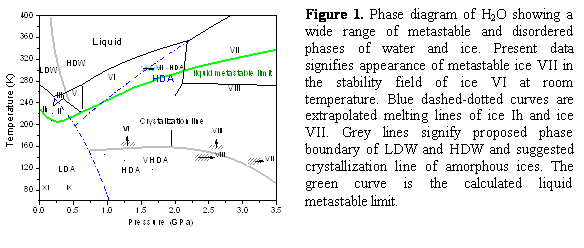
| Figure 1. Phase diagram of H2O showing a wide range of metastable and disordered phases of water and ice. Present data signifies appearance of metastable ice VII in the stability field of ice VI at room temperature. Blue dashed-dotted curves are extrapolated melting lines of ice Ih and ice VII. Grey lines signify proposed phase boundary of LDW and HDW and suggested crystallization line of amorphous ices. The green curve is the calculated liquid metastable limit.
|
 The
phase diagram of water is highly unusual and complex with a wide range of
polymorphs, both stable and metastable, as well as
proton-ordered or disordered forms (Fig. 1). The richness of the phase diagram
attests the versatility of hydrogen bonded network structures including several
forms of “thermodynamically stable” amorphous phases of ices. Information on
amorphous solids, however, is rarely available especially for the stability field
and transformation dynamics–but all reported to exist below the crystallization
temperature of ~150-170 K below 4-5 GPa. Here, we present evidence of high density
amorphous (HDA) ice formed well above the crystallization temperature at 1 GPa–well
inside the so called “no-man's land”. It is formed from metastable ice VII in the
stability field of ice VI under rapid compression using dynamic-diamond anvil cell (d-DAC), and results from structural similarities between HDA and
ice VII. The formation follows an interfacial growth mechanism unlike the
melting process. Nevertheless, the occurrence of HDA along the extrapolated
melt line of ice VII resembles the ice Ih-to-HDA transition, indicating the
structural instabilities of parent ice VII and Ih drive the pressure-induced
amorphization.
The
phase diagram of water is highly unusual and complex with a wide range of
polymorphs, both stable and metastable, as well as
proton-ordered or disordered forms (Fig. 1). The richness of the phase diagram
attests the versatility of hydrogen bonded network structures including several
forms of “thermodynamically stable” amorphous phases of ices. Information on
amorphous solids, however, is rarely available especially for the stability field
and transformation dynamics–but all reported to exist below the crystallization
temperature of ~150-170 K below 4-5 GPa. Here, we present evidence of high density
amorphous (HDA) ice formed well above the crystallization temperature at 1 GPa–well
inside the so called “no-man's land”. It is formed from metastable ice VII in the
stability field of ice VI under rapid compression using dynamic-diamond anvil cell (d-DAC), and results from structural similarities between HDA and
ice VII. The formation follows an interfacial growth mechanism unlike the
melting process. Nevertheless, the occurrence of HDA along the extrapolated
melt line of ice VII resembles the ice Ih-to-HDA transition, indicating the
structural instabilities of parent ice VII and Ih drive the pressure-induced
amorphization.B.
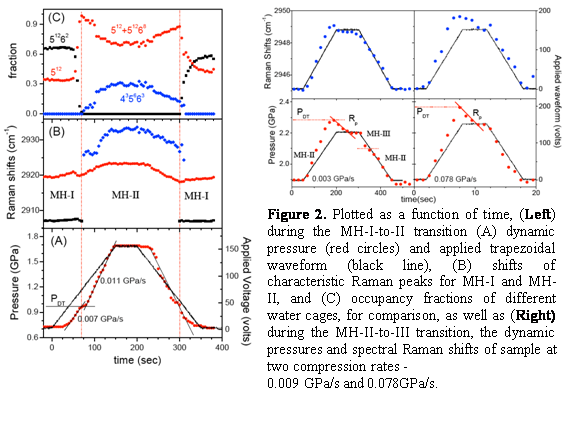
| Figure 2. Plotted as a function of time, (Left) during the MH-I-to-II transition (A) dynamic pressure (red circles) and applied trapezoidal waveform (black line), (B) shifts of characteristic Raman peaks for MH-I and MH-II, and (C) occupancy fractions of different water cages, for comparison, as well as (Right) during the MH-II-to-III transition, the dynamic pressures and spectral Raman shifts of sample at two compression rates - 0.009 GPa/s and 0.078GPa/s.
|
 Compression
dependent kinetics on formation and phase transitions of methane hydrates:
Compression
dependent kinetics on formation and phase transitions of methane hydrates:
We describe high-pressure kinetic studies of formation and phase transitions of methane hydrates (MH) under dynamic loading conditions and over a large range of compression rates, using a dynamic-diamond anvil cell (d-DAC) coupled with time-resolved confocal micro-Raman spectroscopy and high-speed microphotography. The time-resolved spectral and dynamic pressure responses exhibit profound compression-rate dependences associated with both the formation and the solid-solid phase transitions of MH-I to II and MH-II to III (Fig. 2). Under dynamic loading conditions, MH forms only from super-compressed water and liquid methane in a narrow pressure range between 0.7 and 1.6 GPa at the 1D growth rate of 42 mm/sec. MH-I to II phase transition occurs at onset of water solidification 0.9 GPa, following a diffusion controlled mechanism. We estimated the activation volume to be -109±29 Å3, primarily associated with relatively slow methane diffusion which follows the rapid interfacial reconstruction, or martensitic displacements of atomic positions and hydrogen bonds, of 51264 water cages in MH-I to 4351263 cages in MH-II. MH-II to III transition, on the other hand, occurs over a broad pressure range between 1.5 and 1.9 GPa, following a reconstructive mechanism from super-compressed MH-II clathrates to a broken ice-filled viscoelastic solid of MH-III. It is found that the profound dynamic effects observed in the MH formation and phase transitions are primarily governed by the stability of water and ice phases at the relevant pressures.
C. High-pressure kinetics of water solidification in dynamic-DAC:
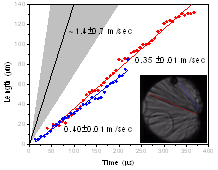
The nucleation and growth process governing the liquid-solid and solid-solid phase transitions strongly depends on homogeneity of solids. The structures of gas hydrates are quite different from ices, yet guest molecules are enclathrated inside hydrogen bonded water cages. Hence, the presence of small impurity molecules can affect strongly the behaviors of hydrogen bonding and alter the phase diagram and phase boundaries of H2O. In this study, we found the effect of methane impurity on solidification and melting of H2O under dynamic loading conditions. The presence of impurity mainly affects the growth rate of metastable ice VII, slowing down from 1.4 m/s in pure H2O to 0.26 m/s in impure H2O, neither the occurrence of metastable ice VII nor the melting of ice VI (Fig. 3).
Figure 3. High-speed microphotographic images of sample during rapid compression showing (A) pure water solidification to ice VII and to HDA in comparison with (B) dirty water solidification to ice VII. The growth rates of metastable ice VII are illustrated on the right, ~ 1.4 m/s (black line) from pure water and ~ 0.35/0.40 m/s (red and blue lines) from dirty water.
SIGNIFICANCE
The present high-pressure kinetic studies provide insight into hydrogen bonding, stabilities, formations, and phase transitions in methane hydrates and water as well as new “chemistry-based” views of high-pressure phase transformations. While the present study is limited to methane clathrates and water, the scientific approach and knowledge are applicable to other gas hydrates including hydrogen hydrates–a new class of hydrogen storage materials. In addition to hydrates, water is such an important element, information obtained regarding high-density amorphous ice and metastable ice phases are of interest to nearly all fields of science and are significant in understanding hydrogen bonding in particular.