www.acsprf.org
Reports: UNI549523-UNI5: Identification of Photocatalytically Active Surface Sites on Layered Transition Metal Sulfides
Tykhon Zubkov, PhD , Ball State University
When dispersed on a nanometer scale, layered sulfides MoS2 and WS2 can exhibit photocatalytic properties.1-4 Hypothesis: the edge sites on the atomic layers dominate the photochemistry. The progress involved several stages:
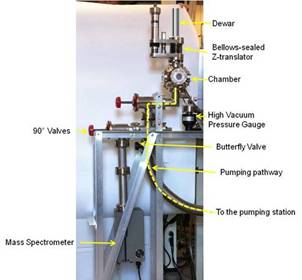
1. Construction of the high-vacuum setup for transmission infrared spectroscopy of surface species has been fully completed using commercial and custom-made parts (Fig. 1). The setup contains two equivalent vacuum cells, which alternatively share Bruker Tensor 27 FTIR spectrometer using a specifically designed nitrogen-purged optical bench with a system of mirrors. The cells allow for IR spectroscopy on powdered samples under simultaneous UV-visible irradiation if needed. This setup is the first of its kind at Ball State University. (Funding: ACS PRF and Lilly V start-up grant.)
Fig. 1. Setup for transmission IR spectroscopy of adsorbates: side view of the high vacuum system and front view with all components assembled.
2. Transmission infrared spectroscopy of surface species on MoS2 and WS2.
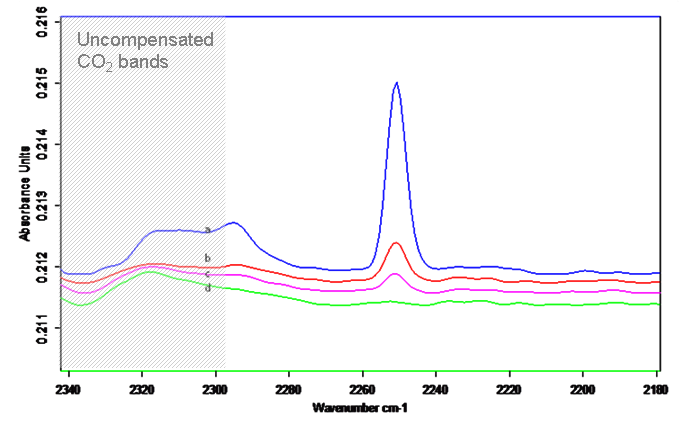
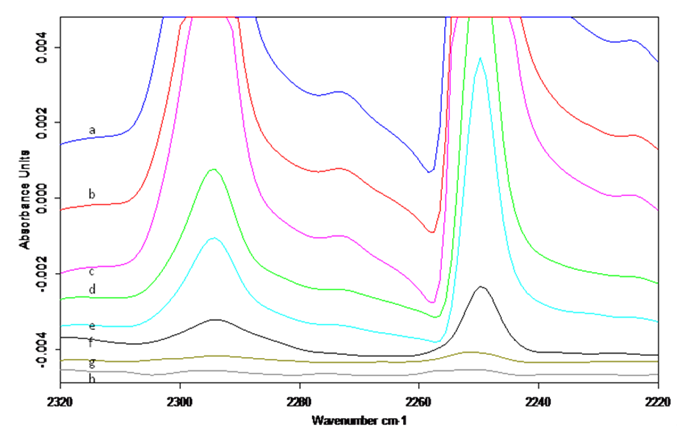
The goal is to study how organic molecules adsorb on specific surface sites and to monitor their chemical transformations. As a proof of concept, adsorption of small molecules was studied on the MoS2 and WS2 nanoparticles and the bulk sulfides. To have site-sensitive vibrational frequency, molecules containing multiple bonds at the terminal atom were used. At 127 K, we could not spectroscopically detect the adsorption of CO, N2, or acetone on these sulfides. The adsorption was apparently too weak to retain these molecules on the sulfides in high vacuum. When using acetonitrile, a less volatile compound, the adsorption was successfully recorded in the spectra. IR bands of adsorbed acetonitrile increase with increasing doses (Fig. 2). In the depletion experiments, the sulfides with pre-adsorbed acetonitrile were thermally annealed to various temperatures and the depopulation of surface sites monitored by IR. At the lowest coverages, the CºN vibrational frequency exhibits a shift from 2250 to 2252 cm-1, indicating the presence of the minority surface sites. While the majority species could be the multilayer acetonitrile ice, it is unclear if the minority species are on the atomic basal planes or on the layer edges. Further studies are needed to definitively assign the spectral features observed.
Fig. 2. IR spectra of acetonitrile adsorbed on the WS2 nanoparticles: (Left) Accumulation upon increasing exposure. (Right) Depletion upon annealing to various temperatures from 127 K to 233 K.
3. Exfoliation of the MoS2 and WS2 atomic layers to produce quasi-two-dimensional sheets.
Exfoliation of atomically thin sulfide sheets of systematically varying width is of interest for catalytic and photocatalytic studies. The charge carriers in the sheets are essentially restricted to a two-dimensional diffusion. As the sheet span increases, the ratio between the edges and planes changes. As starting materials we used MoS2 and WS2 micron-sized particles (commercial products) and the MoS2 and WS2 nanoparticles in the 10-50nm range synthesized surfactant-free. Before exfoliation, we attempted to separate the particles by size. Size exclusion chromatography on a polystyrene column was unsuccessful; the particles permanently attached to polystyrene. Gravity sedimentation fractionation had limited success. The materials were exfoliated using n-butyllithium.5,6 For MoS2, exfoliation proceeds well for both bulk and nanoparticles. For WS2, there is a sharp contrast; bulk WS2 does not exfoliate with n-butyllithium whereas nanoparticles do. This is likely due to the size effect on the interlayer adhesion forces. (Funding: ACS PRF and Indiana Academy of Science.)
4. Synthesis of the MoS2 and WS2 nanotubes for adsorption studies.
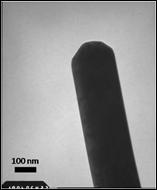
MoS2 and WS2 nanotubes and fullerenes can be prepared by high-temperature sulfidation of the MO3 oxides.7 Lamellar structure is formed while preserving the overall shape of the original oxide particle. Here we report the synthesis of the MoS2 nanotubes from the MoO3 whiskers. The precursor MoO3 whiskers were prepared by sonochemically assisted reduction of peroxomolybdates.8 Subsequent annealing in H2S/H2 atmosphere yields almost exclusively MoS2 nanorods with the typical width of 60-250nm and typical length of 2-8 microns. For WS2, the yield of nanorods is much lower. The rods appear to have a solid core, likely residual oxide. By treating with a base, we were able to etch the cores away to produce hollow nanotubes (Fig. 3). Currently, we aim at producing open ended sulfide nanotubes for adsorption spectroscopic studies.
Fig. 3. Transmission Electron Microscopy images. MoO3 whiskers (left) upon high-temperature sulfidation convert into solid-core MoS2 nanorods (middle). The presumably oxide solid core can be etched with a base to produce hollow MoS2 nanotubes (right).
Securing External Funding
Results obtained with the support of the ACS PRF grant enabled my participation in the Ball State University interdisciplinary proposal to secure an award from NSF in the amount of $497,500. Award Date: 8/22/2011. Award No.DBI-1126196. Project title: "MRI: Acquisition of a Transmission Electron Microscope for Interdisciplinary Research and Teaching." Authors: D.Bishop, B.Pederson, R.Bernot, S.McDowell, T.Zubkov.
Impact on Students
Undergraduate:
· Matthew Bailey wrote his Honors Thesis based on his research. Presented a poster at the ACS Indiana Section poster session in October 2010. Obtained his B.S. in Chemistry and entered the chemistry graduate program at the University of Notre Dame.
· Matthew Crosley presented his research as posters at the ACS Indiana Section poster sessions in October 2009 and 2010, at the 125th Meeting of Indiana Academy of Science in October 2009, and at the 239th ACS National Meeting in March 2010. Received the Smith-Litten-Keys award for his poster at the 2010 Student Research Symposium at Ball State University. Obtained his B.S. in Chemistry and entered the chemistry graduate program at the University of Oklahoma.
· Christopher Lambert and Thomas Shrack will be presenting their research at the ACS Indiana Section poster session in October 2011.
Graduate (Masters program):
· James Leroy wrote his M.S. thesis based on his research. Presented a poster at the 42nd Central Regional Meeting of ACS in June 2011.
· Ryan Combs is currently finalizing his research that is the basis for his upcoming M.S. thesis. Presented a poster at the 42nd Central Regional Meeting of ACS in June 2011.
1.Wilcoxon,J.P.;Samara,G.A.Phys.Rev.B1995,51,7299-7302
2.Thurston,T.R.;Wilcoxon,J.P.J.Phys.Chem.B1999,103,11-17
3.Wilcoxon,J.P.J.Phys.Chem.B2000,104,7334-7343
4.Ho,W.;Yu,J.C.;Lin,J.;Yu,J.;Li,P.Langmuir 2004,20,5865-5869
5.Joensen;Frindt;Morrison.MaterRes.Bull.1986,21, 457
6.Heising;Kanatzidis.J.Am.Chem.Soc.1999,121,1720-11732
7. Tenne,R.;Homyonfer,M.; Feldman,Y.Chem.Mater.1998,10,3225-3238
8. Chu,B;Krishnan,C.V.;Chen,J;Burger,C; J.Phys.Chem.B2006,110,20182-20188