www.acsprf.org
Reports: ND748942-ND7: Phase Transitions in Mixtures of Micelles and Polyelectrolytes: Electrostatic Assembly of Soft Colloids
Anthony D. Dinsmore , University of Massachusetts (Amherst)
P. L. Dubin , University of Massachusetts (Amherst)
We have performed experiments to elucidate the mechanisms that drive phase separation in mixtures of polyelectrolytes and oppositely-charged particles. Mixtures of this kind are common in industrial settings e.g., in tertiary oil recovery, in cosmetics, and in materials for drug delivery. Many years of experiments have shown that these mixtures can undergo phase separation, often into two coexisting liquid phases. This phase separation is known as coacervation and is thought to arise principally from electrostatic and entropic interactions that lead to a complex of positively and negatively charged molecules and particles. The overall behavior of these systems depends on many parameters and an overall understanding has so far eluded researchers.
The goal of our experiments is to characterize the structures that appear near these phase transitions. Previous reports have shown that very large aggregates (up to 100 nm in size) appear spontaneously in the sample even in the single-phase region. These aggregates are stable in suspension and form reversibly: they are in equilibrium. We have measured the size (RH) and concentration of these aggregates throughout the phase diagram and determined empirically how they depend on polymer molecular weight, charge stoichiometry, concentration, and temperature. We seek to establish a connection between the size of these aggregates and the structure of the new phase that emerges during coacervation. We are motivated by the hypothesis that the aggregates can be viewed effectively as colloidal particles, and that coacervation may be viewed as phase separation of these “colloidal” particles. By this hypothesis, the coacervate would contain density variations over a size scale similar to the size of aggregates in the coexisting dilute liquid phase.
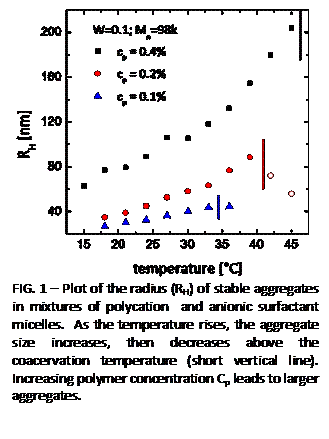
| FIG. 1 - Plot of the radius (RH) of stable aggregates in mixtures of polycation and anionic surfactant micelles. As the temperature rises, the aggregate size increases, then decreases above the coacervation temperature (short vertical line). Increasing polymer concentration Cp leads to larger aggregates. |
 Our
experiments are done with mixtures of a polycation
and anionic micelles with tunable surface charge density.
Characterization is primarily done using static and dynamic light scattering,
neutron scattering, and optical microscopy. We systematically explore the
roles of temperature, concentration of cationic and anionic species, and
polymer molecular weight.
Our
experiments are done with mixtures of a polycation
and anionic micelles with tunable surface charge density.
Characterization is primarily done using static and dynamic light scattering,
neutron scattering, and optical microscopy. We systematically explore the
roles of temperature, concentration of cationic and anionic species, and
polymer molecular weight. An important result is that the size of the aggregates (RH) increases as the phase transition is approached and that the size varies smoothly across the transition (Fig. 1). Depending on the composition, RH can exceed 200 nm, which is far larger than the size of an individual micelle or polymer (~35 nm).Remarkably, the sizes of these aggregates are stable; they do not continue growing to macroscopic size. Images obtained with total-internal reflection fluorescence microscopy (TIRF) allow us to identify these aggregates directly, showing that they exist as discrete entities. (These experiments were done with our colleague, Prof. M. D. Barnes of UMass Chemistry.)
We have probed the structure within the concentrated phase (also known as the coacervate phase). Using small-angle neutron scattering, we found characteristic length scales on the order of 100 nm, a length that is comparable to the size of the aggregates that would be expected in the coexisting dilute phase (supernatant). These results are consistent with the hypothesis that the coacervate is a concentrated fluid of aggregates.
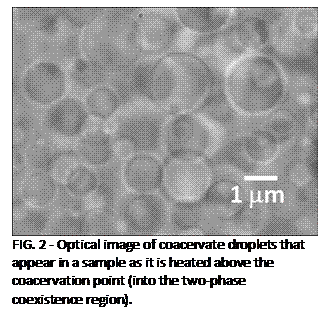
| FIG. 2 - Optical image of coacervate droplets that appear in a sample as it is heated above the coacervation point (into the two-phase coexistence region). |
 By
monitoring samples in an optical microscope during heating, we visualized the
phase separation process directly.
Images show that the concentrated (coacervate)
phase appears as spherical droplets that have well-defined boundaries (Fig. 2). The smallest droplet we have seen are
approximately a micron in radius. Assuming
that the droplets form by the classical nucleation process, this observation places
an upper bound of 1 micron, or approximately ten aggregate diameters, on the
critical droplet radius.
By
monitoring samples in an optical microscope during heating, we visualized the
phase separation process directly.
Images show that the concentrated (coacervate)
phase appears as spherical droplets that have well-defined boundaries (Fig. 2). The smallest droplet we have seen are
approximately a micron in radius. Assuming
that the droplets form by the classical nucleation process, this observation places
an upper bound of 1 micron, or approximately ten aggregate diameters, on the
critical droplet radius. This project has provided rigorous training for our Postdoctoral Fellow, Simona Maccarrone, graduate student Ebru Kizilay, and undergraduate student Elaine Foun. These students received interdisciplinary training in a collaboration between faculty in the Physics and Chemistry departments at UMass Amherst. This project is synergistic with the Prof. Dinsmore's growing interest in cluster formation in systems that have long-range repulsive interactions that compete with short-range attraction. Two new projects have recently been begun, which are synergistic with this New Directions grant. One explores the effect of these competing interactions among colloidal spheres, where the interactions can be controlled. The other investigates formation of very large yet stable aggregates as a multi-component suspension is dried to form a thin film.
