www.acsprf.org
Reports: B147559-B1: Diels-Alder Reactions of Silyloxy Furans: Scope and Limitations
Scott K. Bur , Gustavus Adolphus College
Progress for this granting period
Duringthis granting period, we cleaned up result from the last reporting period andsubmitted a manuscript to Tetrahedron Letters. Our synthetic effortswere divided between two major areas: continuing investigations of theintermolecular reactions and using what we learned from the intermolecularreactions to build substrates for intramolecular reactions.
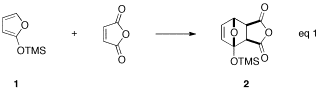
Withthe intermolecular reactions, we became particularly interested in thechemistry of the cycloadducts, which arise from the reaction of TMSO-furan (1) and maleic anhydride (eq 1), dimethyl maleate, ordimethylfumerate.
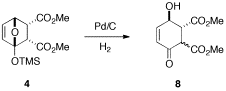
For example, Reaction with LiAlH4with 2 was expected to produce tetraol 3 (Scheme 1). Unfortunately, analysis of the 1H NMR spectra of the crudereaction mixture revealed no evidence of either 2 or 3. No reaction occurred between 2 and NaBH4, and both CaBH4 andLiBH4 return intractable mixtures. Similar results were observed for the reactions of 4, 6,and 7.
Wenext turned our attention to other types of reductions under milderconditions. The palladiumcatalyzed hydrogenation of 4 provided 8quantitatively (eq 2). It was clear by both TLC and NMRanalysis of the crude reaction mixture at various time points thatisomerization of the b-ketoesterstereochemistry was equilibrating throughout the reaction, and attempts toisolate pure diastereomers were unsuccessful. These studies continue.
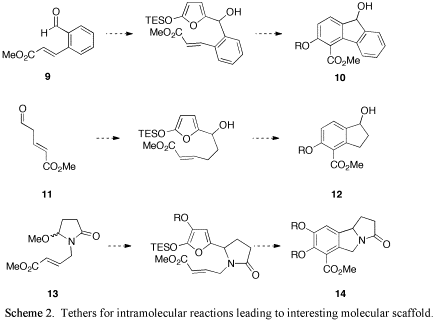
Withan understanding of the intermolecular reaction dynamics, we turned ourattention to designing substrates for the intramolecular reactions thatincorporate features we identified as critical. To that end, we have started building suitable tethers withelectron-withdrawing groups conjugated to the dienophilic moiety. For example, 2-iodobenzoic acid wasconverted into 9 (Scheme 2). We also synthesized known aldehyde 11 and hydroxylactam 13.
Each of these starting materialsis expected to provide access to a different type of interesting corestucture. Aldehyde 9, for example could provide access to fluorenolderivative, aldehyde 11 willlead to indanes (and, depending upon the substitution pattern, a variety ofterpene natural products.), and lactol 13 could open the methodology up to nitrogen-containing heterocycles like14, which represents the core ofthe erythrinia alkaloids.
Overall impact of the grant
This grant has opened up a new avenue of research at GustavusAdolphus College, and has attracted much student interest. On an institutional level,this grant has been part of a successful effort to increase undergraduateresearch opportunities in the summers. In combination with two institutional grants (Merck Institute forScience Education and Howard Hughes Medical Institute), the PRF grant hashelped alter the research atmosphere at Gustavus profoundly.
Seven students were involved forvarious lengths of time, five of them women. Three are attending graduate school (two for chemistry, onefor biochemistry), one is attending medical school, one entered the workforce(3M), and two are still students at Gustavus. One of the students attended a national ACS meeting topresent results of this work. The connectionhe made at the meeting weighed heavily in his choice of graduate program.
The PI was awarded tenure, partlybecause of the work accomplished early in the granting period. It has also provided the PIseveral examples with which to enrich his teaching. The fluorenol result (e.g. 10) has the potential to move this particular project well away fromnatural product applications and into areas that are fundamentally new to thePI. We are initiating acollaboration with a physical organic chemist to study the spectroscopicproperties of these molecules has begun.