www.acsprf.org
Reports: DNI1049002-DNI10: Tailorable Organic Nanoscale Piezoelectrics for Energy Harvesting
Geoffrey R. Hutchison, PhD, BA , University of Pittsburgh
Background and Significance
Piezoelectrics are polar materials that produce electrical charge in response to a mechanical distortion, or change shape in response to an applied electric field. As such, they have achieved wide use as combined sensors and actuators for applications as wide-ranging as sensors, ultrasonic motors, scanning-probe microscopy manipulation, and airbag impact sensors. A variety of conventional piezoelectric bulk materials are known, including toxic perovskite materials such as lead zirconium titanate (Pb[ZrxTi1-x]O3 or PZT), poly(vinylidinedifluoride) (PVDF) and quartz. Work by other groups has shown that zinc oxide (ZnO) wires combine semiconducting and piezoelectric properties for micropower generation, including hybrid fabrics generating up to 20 mW/m2.
These ZnO devices illustrate the promise of piezoelectric materials for energy harvesting, but they also reflect a balance between piezoelectric response and conductivity. For example, high piezoelectric coefficients are found in PZT, which is an insulator, while ZnO has higher conductivity but lower piezoelectric response. Consequently, we are investigating the use of designed molecular materials to yield both high piezoelectric response and conductive properties.
In our first year, we demonstrated predictions of the piezoelectric deformation of single molecules, and studied substituents and regioisomers to produce initial structure/property relationships. Using these design rules, we synthesized our polar helicene target.
In the second period of support, we have refined our structure/property relationships using a new, more synthetically accessible family of piezo-active molecules, and have for the first time, measured the piezo response of single monolayers of oligopeptides.
Computational Study
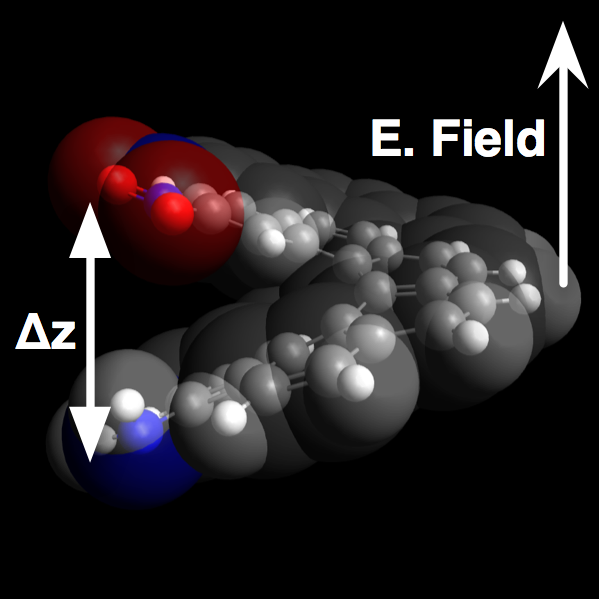
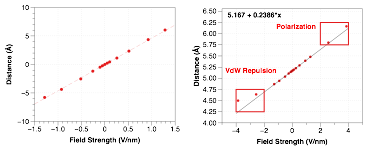
Over the course of the funding period, have demonstrated that individual molecules can significantly change geometry under an applied electric field. Using Gaussian, we assert an external electric field with varying strength along the long axis of helical molecules to force conformational changes, as illustrated in Figure 1. The optimized geometry as a function of applied field clearly illustrate the piezoelectric effect in Figure 2. For this particular compound, 4-amino-5-nitro[6]helicene (4a15n), the computed deformation of 12% corresponds to a piezoelectric coefficient (d33) of 45.8 pm/V, greater than ZnO or PVDF.
Figure 2. (Left) Calculated N to N distances in 4a15n under different applied external electric fields. (Right) While the deformations are largely linear across the field strengths studied, some curvature does occur at high positive and negative applied fields.
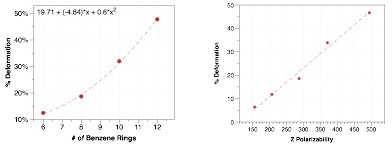
We considered substituent effects and different regioisomers of the helicenes to establish important correlations between molecular structure and piezoelectric deformation. Surprisingly, the dipole moment of the molecule has low correlation with the piezo response, even though the product of the applied field and dipole moment yield the potential energy difference that distorts the lowest-energy geometry. Instead, the induced dipole moment (and thus the polarizability) of the molecule are the most important contribution to the piezoelectric coefficient. As we increase the length of the conjugated system, we increase the number of benzene rings and polarizability, directly correlating with increased piezoelectric response, as seen in Figure 3.
Figure 3. (Left) Computed piezoelectric deformation as a function of the number of benzene rings in the aromatic system (i.e., 6 for [6]helicene). (Right) Direct correlation between the polarizability of the aromatic molecule and the computed piezo deformation.
We have used these calculations and structure-property relationships to derive a new family of piezoactive molecules with increased response over our initial helicenes. The smallest member is predicted to yield 18% greater piezoresponse over our [6]helicene (d33 = 59 pm/V, versus 45.8 pm/V for 4a15n).
Experimental Characterization
 In
addition to computational studies over the first year of the project, we have
experimental verification of the single-molecule piezoelectric effect using piezo-response AFM measurements of self-assembled
monolayers (SAMs). We use simple solution-based microcontact
printing techniques to deposit and SAMs on smooth gold films through the
cysteine thiol-gold interaction, as illustrated in
Figure 4. Using piezo-force AFM techniques to image
both the topography (height) of our films, and the piezoresponse, individual
peptide molecules deform significantly in response to the applied electric
field between the tip and surface. Figure 5 illustrates PFM images for both
topography and piezoresponse for a patterned SAM with an alternating stripe of
(Ala)6-Cys
surrounded by inactive dodecanethiol.
In
addition to computational studies over the first year of the project, we have
experimental verification of the single-molecule piezoelectric effect using piezo-response AFM measurements of self-assembled
monolayers (SAMs). We use simple solution-based microcontact
printing techniques to deposit and SAMs on smooth gold films through the
cysteine thiol-gold interaction, as illustrated in
Figure 4. Using piezo-force AFM techniques to image
both the topography (height) of our films, and the piezoresponse, individual
peptide molecules deform significantly in response to the applied electric
field between the tip and surface. Figure 5 illustrates PFM images for both
topography and piezoresponse for a patterned SAM with an alternating stripe of
(Ala)6-Cys
surrounded by inactive dodecanethiol.
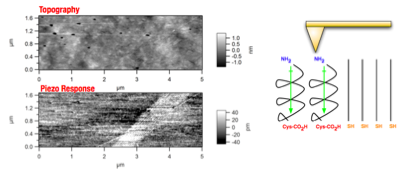
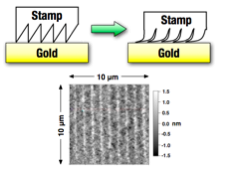
Figure 4. (top) Stamping of self-assembled monolayers (SAMs) of piezoelectric molecules on ultra-flat gold. (bottom) Atomic-force microscopy (AFM) of oligopeptide aggregates (bright spots) across a 10 μm gold film, showing 1.0 μm pattern.
Figure 5. (left) AFM topography of a patterned, SAM on a smooth gold film with Cys-(Ala)6 oligopeptide surrounded by dodecanethiol (right). Since both molecules are ∼9 tall, no height contrast is observed. However, with piezoresponse (PFM) using a driving voltage of 3V, clear contrast is observed between the non-piezoelectric dodecanethiol and the oligopeptide regions.
Over the final period of the funding, we have provided clear structure/property relationships from our computational studies, demonstrating significant piezoelectric response for organic molecules using conformational changes under an applied electric field. We synthesized several target compounds shown to have high response from our computational studies, found a new family of active molecules, and are undertaking experimental characterization of the piezoelectric response in thin-films and monolayers.
Impact of PRF Award
The award for this research under the DNI program was the first outside research funding received by the Hutchison group. Consequently, it allowed us to pursue a novel, risky research direction with little preliminary results. In response, we drove this project from concept to detailed structure/property relationships, synthesis, and experimental verification. The award has resulted in two manuscripts: a computational study submitted to Journal of Physical Chemistry C, and the experimental characterization of single monolayers, to be submitted to Nature Nanoscience this fall. The graduate student supported by this project has showed tremendous promise, completing the computational simulations, developing and executing the synthetic route to the bi-substituted [6]helicenes, and testing piezo AFM and device characterization techniques. We strongly believe that thanks to the ACS PRF support, this new research area will easily expand into novel energy harvesting applications and new areas of chemistry. Our preliminary results have resulted in interest from several funding agencies, including the DOE and AFOSR.