www.acsprf.org
Reports: DNI349296-DNI3: Structural and Functional Modeling of Nickel Superoxide Dismutase
Michael Jensen, Ph.D. , Ohio University
1. Introduction. Recent structure determinations of nickel-dependent enzymes have established new paradigms for biological redox processes. For example, the (N3S2)3- ligand field of nickel-dependent superoxide dismutase (NiSOD) supports a facile Ni(II/III) couple. Axial ligation of the His-1 sidechain is thought to be essential in stabilizing the high-valent state in a penta-coordinate geometry at the N-terminal active site of the protein. With support of the Petroleum Research Fund, we prepared a series of bio-inspired Ni(II) complexes incorporating hydrotris(3-phenyl-5-methylpyrazol-1-yl)borato ligands with anionic chelating dithiocarbamate co-ligands, R2NCS2- (i.e., TpPh,MeNiS2CNR2). These complexes were designed to exhibit biomimetic axial base equilibria through variable k2- and k3-ligation of the tripodal ligand. We observed axial base equilibria with concomitant spin crossover of Ni(II) between diamagnetic (S = 0) and paramagnetic (S = 1) electronic states, as well as quasi-reversible one-electron redox couples We have also examined reactivity with the superoxide substrate.
2. Impact. One graduate student supported with PRF funds, Dr. Huaibo Ma, completed his Ph.D. and assumed a postdoctoral appointment at Emory University. Two other doctoral students were supported on a part-time basis, and will continue their dissertation research during the recently approved grant extension. One manuscript acknowledging PRF support was published this year (H. Ma, et. al J. Am. Chem. Soc. 2011, 133, 5644-5647), and additional manuscripts currently under preparation will be submitted during the grant extension. Our results were also used to support a pending application to the National Science Foundation. Finally, the travel funds enabled us to present results at several regional, national and international meetings in the past year, including CERMACS 2011 (Abstract #200), the 240th ACS National Meeting (INOR 687), and Pacifichem 2010 (Inorganic 2-1490). PRF support had a vital impact on productivity, graduate training, dissemination of results and future funding.
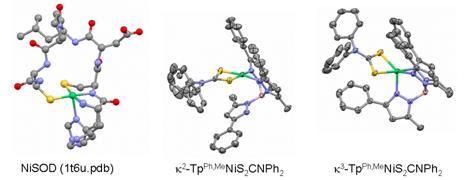
3. Results. X-ray crystallography revealed both square-planar N2S2 and square-pyramidal N3S2 geometries at the NiSOD active site, respectively assigned Ni(II) and Ni(III) oxidation states (1t6u.pdb: D. B. Barondeau, et al. Biochemistry 2004, 43, 8038-8047), and distinguished by disposition of the His-1 sidechain with respect to an axial coordination site (Figure 1, left). Our complexes exhibit a similar axial equilibrium involving one arm of the TpPh,Me ligand (Figure 1, center and right), at Ni(II).
Figure 1. Comparison of NiSOD structures (left) with model complexes (center, right).
The k2 isomer is square-planar and diamagnetic, while the k3-isomer is paramagnetic (S = 1) and exhibits significant trigonal distortion (t = 0.61). These equilibrate readily in solution (DGo298K ≈ 0.5 kcal/mole), and addition of equivalent acid drives the k2/k3 equilibrium towards the conjugate acid of the square-planar structure (by analogy to four-coordinate reduced NiSOD, wherein both nitrogens of the detached imidazole are thought to be protonated).
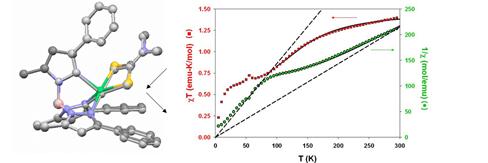
In the case of the TpPh,MeNiS2CNMe2 analog, we discovered spin equilibrium in the solid state; to the best of our knowledge, this is the first structurally characterized spin crossover of Ni(II).The unit cell contained independent five-coordinate molecules; one of these exhibited a significant axial Ni-N bond elongation between 293 K (2.15 Å) and 123K (2.40 Å), while the other remained short (2.05 and 2.04Å, respectively), consistent with high-spin (S = 1) Ni(II). Modulation of the axial bond length gives rise to spin equilibrium selectively at the first Ni(II) center. Magnetization data obtained in collaboration with Prof. Gordon Yee of Virginia Tech confirmed equilibrium of half the spins (DH° = kcal/mol, DS° = cal/molK; T½ = 155 K, Figure 2). From the calculated equilibrium positions, it was possible to extract limiting bond lengths for the elongated, diamagnetic square pyramidal N3S2 ligand field.
Figure 2. Least-squares overlay of x-ray structures at the crossover site in the lattice of TpPh,MeNiS2CNMe2 (left), at 293 K (color) and 123 K (grayscale); solid-state magnetization data (right), with the least-squares equilibrium fit for half the spins over a 100-300K range (solid black curves).
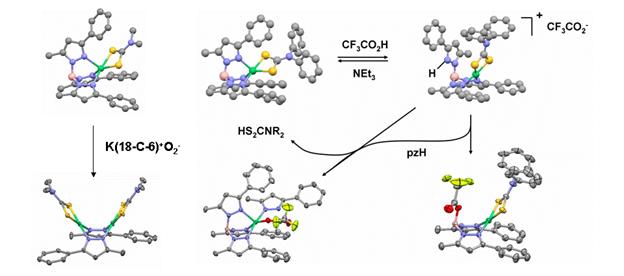
We were also interested in functional mimicry of the NiSOD active site, and therefore determined products obtained by protonation and superoxide addition (Figure 3). The latter substrate rapidly attacked the boron atom of TpPh,MeNiS2CNMe2, disrupting the tripodal chelate to form a three-coordinate (dithiocarbmato)(pyrazolato)Ni(II) fragment that undergoes head-to-tail dimerization to yield [(Me2NCS2)Ni(1,2-m-3-Ph-5-Mepz)]2. While protonation of TpPh,MeNiS2CNR2 complexes is immediately reversible, the conjugate base can also attack at boron, displacing the protonated axial pyrazole to form the structurally characterized acidolysis product [{F3CC(O)OB(H)(3-Ph-5-Me-pz)2}NiS2CNPh2]. The displaced pyrazole can then attack a second equivalent of protonated complex, which results in displacement of dithioacid from nickel(II).
Figure 3. X-ray structures of decomposition products arising from addition of superoxide (left) and acid (right) in CH3CN solutions at room temperature.
Functional mimicry was circumvented by the striking electrophilicity of boron. We prepared tris(pyrazolyl)methane complexes to address this problem, but the weaker ligand field favored octahedral coordination without an observable axial base equilibrium (e.g. [k3-TpmMe,MeNi(S2COEt)Cl]). Nevertheless, substitution of boron to form k2-bis(pyrazolato) chelates has enabled us to examine the effects of axial base removal on the electrochemistry of rigorously square-planar complexes.
4. Future directions. During the recently approved grant extension, we will prepare and submit manuscripts describing our results, which extend beyond the summary herein. With remaining support, we will also seek suitable conditions to chemically oxidize these complexes without disruption of the TpPh,Me tripod, in order to prepare and characterize high-valent intermediates. Moreover, we will modify the dithiocarbamate substituents to examine prospects for using our robust Ni(II) complexes as spin crossover materials exhibiting unique behavior arising from intermolecular interactions (e.g., hydrogen bonding, p-stacking, etc.) and large axial ligand field distortions.