www.acsprf.org
Reports: UNI349424-UNI3: Rational Design and Synthesis of Structural Analog Complexes of the Active Site of Ni-Fe Hydrogenases
JIanfeng Jiang, PhD , Yeshiva University
Introduction
We proposed to design and synthesize analog complexes of the active sites of Ni-Fe hydrogenases.
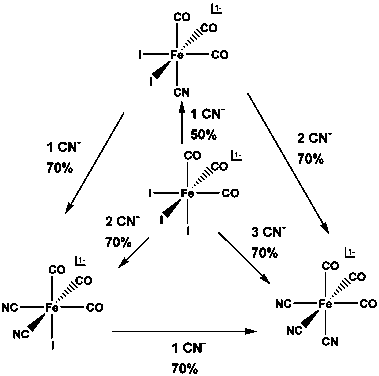
In the second funded year we have studied the substitution reaction of fac-[Fe(CO)3I3]- by 1, 2 or 3 equivalents of cyanides. By controlling the reaction conditions, we were able to stepwise replace iodide by cyanide and isolate fac-[Fe(CN)x(CO)3I(3-x)]- (x = 1, 2 and 3). A manuscript describing these substitution reactions was submitted to Inorganic Chemistry Communications and currently under review.
We have tried different thiolates and phosphines using the procedure leading to the isolation of (dppe)Ni(mu-pdt)Fe(CN)2(CO)2, (pdt = 1,3-propanedithiolate and dppe = 1,2-bis(diphenylphosphino)ethane) and studied the spectroscopic trend of the products and this study is currently in progress.
In order to illustrate the structure and isomerization of [Fe(CN)2(CO)2(SR)x]x- (x = 1 or 2), we have studied the reactions of fac-[Fe(CN)2(CO)3I]- with 1 or 2 equivalents of thiolates. This study is still ongoing.
In addition, we have generated Ni(N2S2)Fe(CN)2(CO)2 type bridging dimer by the reaction of fac-[Fe(CN)2(CO)3I]- with Ni(N2S2) macrocycle type compounds and studied the dimer's reaction with hydride in an attempt to make hydride bridging Ni-Fe analog complexes.
Results
1. Stepwise substitution of iodide by cyanide from fac-[Fe(CO)3I3]1-
Cyanide can stepwise replace iodide from fac-[Fe(CO)3I3]1- to form fac-[Fe(CN)x(CO)3I(3-x)]- (x = 1, 2 and 3). The conversions to fac-[Fe(CN)(CO)3I2]-, fac-[Fe(CN)2(CO)3I]-, and fac-[Fe(CN)3(CO)3]- are 50%, 70% and 70%, respectively. (Scheme 1) fac-[Fe(CN)(CO)3I2]- is a new compound and can be used as excellent starting material to prepare analog complexes for the Fe-Fe hydrogenase active sites since it only has 1 CN and all other CO/I ligands are replaceable. The whole series are structurally characterized and their structural and spectroscopic properties follow nice trend according to the ligands electronic properties.
Scheme 1: The reactions between the fac-[FeII(CN)x(CO)3I(3-x)]- (x = 0 – 2) and cyanide. The percentiles are the conversion ratio in solution, not the isolated yield.
2. Systematic synthesis of Thiolate Bridging Ni-Fe analog complexes with general formula: (PR3)2Ni(mu-SR)2Fe(CN)2(CO)2
In order to illustrate the structure and property relationship of the dithiolate bridging Ni-Fe dimers, we have tried to systematically synthesize compounds with general formula: (PR3)2Ni(mu-SR)2Fe(CN)2(CO)2. There are 2 methods for the synthesis; the first method is from the reaction between [Fe(CN)2(CO)2(SR)2]2- and (PR3)2NiCl2 and the second method is from the reaction between [Fe(CN)2(CO)3I]- and (PR3)2Ni(SR)2. The following table showed the combinations of thiolates and phosphines we have tried.
| pdt2- | edt2‑ | bdt2- | EtS- | 1PrS- | 2PrS- | tBuS- | PhS- | BzS- |
dppe | Y | Y | X | Y | Y | Y | X | Y | Y |
dppp | Y | Y | X | Y | Y | Y | X | Y | Y |
dppv | Y |
|
|
|
|
|
|
|
|
dppbz | Y |
|
|
|
|
|
|
|
|
dmpe |
|
|
|
|
|
|
|
|
|
PPh3 | Y |
|
|
|
|
|
|
|
|
PEt3 | Y |
|
|
|
|
|
|
|
|
PCy3 | Y |
|
|
|
|
|
|
|
|
Table 1: thiolates and phosphines chosen to synthesize compounds with general formula: (PR3)2Ni(m-SR)2Fe(CN)2(CO)2. Y refers to positive reaction, X refers to negative reaction and Void refers to reaction has not been tested. (dppe = 1,2-Bis(diphenylphosphino)ethane, dppp = 1,3-Bis(diphenylphosphino)propane, dppv = cis-1,2-Bis(diphenylphosphino)ethene, dppbz = 1,2-Bis(diphenylphosphino)benzene, dmpe = 1,2-Bis(dimethylphosphino)ethane, PPh3 = triphenylphosphine, PEt3 = triethylphosphine, PCy3 = tricyclohexylphosphine, pdt2- = 1,3-propanedithiolate, edt2- = 1,2-ethanedithiolate, bdt2- = 1,2-benzenedithiolate, EtS- = ethanethiolate, 1PrS- = 1-propanethiolate, 2PrS- = 2-propanethiolate, tBuS- = tert-butanethiolate, PhS- = phenylthiolate and BzS- = benzylthiolate)
We have not finished all the reactions yet.
3. Reaction of fac-[Fe(CN)2(CO)3I]1- with 1 or 2 equivalents of thiolates
The reaction of fac-[Fe(CN)2(CO)3I]1- with 2 equivalents of thiolates showed that the product(s) always has(have) 2 cis-CO. However, the 2 CN can be either cis- or trans-. The 2 thiolates can also be trans- if monodentate thiolates are used. The reaction of fac-[Fe(CN)2(CO)3I]1- with 1 equivalent of thiolate lead the formation of [Fe(CN)2(CO)2(SR)]22- dimer, which can also be generated by the reaction between [Fe(CN)2(CO)3I]1- and [Fe(CN)2(CO)2(SR)2]2-.
We are trying to structurally characterize these products now.
4. Study of the reaction between Ni-Fe analog complexes with hydride
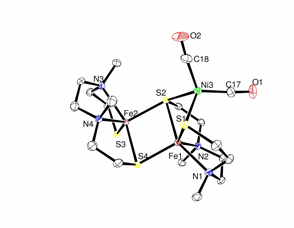
Some of catalytic states of Ni-Fe hydrogenases active sites contain a bridging hydride between Fe and Ni. People believe that the hydride containing states are key states in the formation/consumption of H2. We have attempted to react our Ni-Fe complexes with hydride using lithium hydride triethylborane (LiHBEt3) as hydride source. This study is not successful currently as the hydride addition always led the decomposition of the Ni-Fe dimers. One of the decomposition product was identified crystallographically and published in Acta Crystallography E. The Structure of the decomposed product is shown in Figure 1.
Figure 1: ORTEP drawing of [Fe(dsdm)]2Ni(CO)2 at 50% probability level. (dsdm = dimethyl-3,6-diazaoctane-1,8-dithiolato)
Summary
Stepwise cyano substitution of iodide from fac-[Fe(CO)3I3]- was studied and the whole series of fac-[Fe(CN)x(CO)3I(3-x)]- (x = 1, 2 and 3) was characterized. Thiolates-bridging Ni-Fe compounds with general formula (PR3)2Ni(m-SR)2Fe(CN)2(CO)2 were synthesized as an attempt to illustrate the relationship between structure and properties of the Ni-Fe dimer. Reactions of fac-[Fe(CN)2(CO)3I]1- with 1 or 2 equivalents of thiolates are studied, the products are in the final stage of characterization. Reactions between Ni-Fe dimers and hydride were studied, however, only decomposed products are determined.
We have published 2 papers in this funded year and 1 manuscript is currently under review. More publications will be generated in the near future thanks to the support of Petroleum Research Fund.