www.acsprf.org
Reports: DNI750534-DNI7: Reversibility and Kinetic Trapping in Polymeric Nanostructures
Marina Tsianou, PhD , State University of New York at Buffalo
The goal of this research project is the fundamental understanding of the mechanisms behind phase separation, kinetic trapping, and reversibility in polymeric nanostructures that will in turn empower the rational formulation of such mixtures in a wide range of commercial applications where the efficiency and long-term stability of products play a crucial role. The equilibrium phase behavior and structure of colloidal systems is a direct manifestation of the system free energy, and thus provides access to fundamental information regarding the operating molecular and supramolecular interactions. Frequently, the time required for equilibrium to be attained is rather long, and often is indeterminate.
We focus here on mixtures of polyelectrolytes with oppositely charged polymers or oppositely charged surfactants. Polymers and surfactants are typically employed in tandem for structuring aqueous media. Such structuring is necessary for conferring viscoelastic rheological behavior (a polymer feature) to water, for improving colloidal stabilization, and for enabling a hydrophobic microenvironment (a surfactant feature) suitable for solubilization of molecules otherwise insoluble in water. The solution behavior of each of the components is important, however, the final performance of the formulated product depends to a large extent on the interplay of complex interactions between the polymer and the surfactant molecules. When the polymers and surfactants are mixed together miscibility is typically desired, and demixing, if it occurs, is an unwanted side effect with tremendous consequences on the structure, appearance, and performance of products.
To this end, we have undertaken a systematic investigation of different polymer/polymer or polymer/surfactant systems in order to determine the phase behavior and stability of the non-equilibrium structures formed by these, and assess how easily they can be switched over to other states (one-phase solutions or macroscopically phase-separated mixtures) by perturbations in the concentration of the components or in temperature. We are working with positively charged poly(diallyldimethylammonium chloride) (PDDA), cationic hydroxyethyl cellulose (cat-HEC), hydrophobically modified cationic hydroxyethyl cellulose (HM-HEC) and we investigate the phase behavior when these are mixed with oppositely charged polymers or surfactants including poly(acrylic acid) (PAA), HM-poly(acrylic acid), poly(styrene sulfonate) (PSS) or sodium dodecyl sulfate (SDS) surfactant. We explore the effects of different mixing procedures on the stability of the samples by i) simple mixing of aqueous solutions of the components (in two different pathways: polymer added to surfactant, and surfactant added to polymer), and ii) rapid mixing using a stopped-flow apparatus. Interestingly, our results on systems of HM-cationic hydroxyethyl cellulose and SDS indicate that the way of mixing (adding polymer to surfactant or surfactant to polymer with the same final composition) leads to different-looking samples (in terms of turbidity) and that the state of the system is strongly depended on the order of addition of the components and the way the solution was mixed.. We also compare the initial state of the complexes and follow their kinetics over time by turbidity, density, electrophoretic mobility, and scattering measurements. The focus is on samples that do not clearly show macroscopic phase separation as well as on the characterization of structures near to phase transitions.
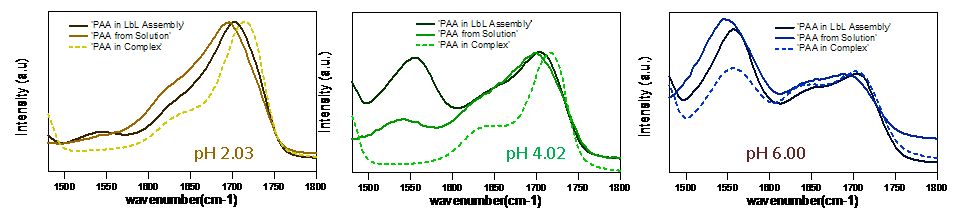
In another system involving PDDA and oppositely charged PAA we are investigating the effect of pH on the PDDA/PAA complex formation and compare this to multilayers formed by subsequent deposition of PDDA and PAA (Layer-by-Layer assembly method). Such studies will allow us to compare the behavior of polyelectrolyte multilayers to that of the corresponding polyelectrolyte complexes in solution. The attached FTIR spectra of PDDS/PAA systems in multilayers and in the corresponding complexes in solution at various deposition pH values of PAA clearly indicate pH depended differences in the charge density of the resulting complexes (the peak around 1690-1710 1/cm corresponds to COOH and peak at 1530-1560 1/cm corresponds to COO-).
Plans for the upcoming year include studies on the time-evolution of complex formation and structure upon mixing of the ingredients, as affected by the molecular characteristics of the species, concentrations, and stoichiometric ratio. Anticipated results are fundamental insights into the metastability of polymer/polymer and polymer/surfactant complexes, and practical pathways to achieve monophasic systems and avoid phase separation in an extensive concentration range of the components.