www.acsprf.org
Reports: UNI1050237-UNI10: Controlling Morphology and Electronic Properties of Two-Dimensional Organometallic Conjugated Polymers via Orthogonal Polymerization Methods
Katsu Ogawa, Ph.D. , California State University (Northridge)
Development of new and efficient materials for organic solar cells has become one of the most important areas of chemistry. The performance of photovoltaic devices highly depends on efficiencies of absorption, charge separation, and charge migration. Our research goal is to improve charge migration efficiency by constructing 2D conjugated polymer matrix. In our approach, phosphole type ligands are incorporated in place of typical trialkyl phosphine ligands for platinum acetylide polymers. Unlike its nitrogen analogue (pyrrole), the lone pair on phosphorus atom is not completely associated with the pi system of the phosphole ring. As a result, phosphole exhibit non-planar structure with ligating capability through the lone pair. On the other hand, phospholes can be polymerized via oxidation just as pyrroles and thiophenes. The introduction of phosphole ligands in Pt-acetylide polymers results in possibility for cross linking a conjugated polymer (Pt-acetylide) with another conjugated polymer (Polyphosphole). Such cross linking should lead to increased conjugation, hence the improved charge migration for enhanced efficiency of the photovoltaic devices.
First Year Accomplishment
So far, our efforts have been focused on synthesis of 1-phenyl-2,5-bis(2-thienyl)phosphole. In the original synthetic scheme, this target molecule was to be synthesized from 1,4-bis(2-thienyl)butadiyne utilizing a literature procedure for synthesis of 1,2,5-triphenylphosphole from 1,4-diphenyl-1,3-butadiyne and dichlorophenylphosphine. Sonogashira coupling reaction between 2-bromothiophene and TMS-acetylene gave TMS protected ethynylthiophene in 96% yield. Then this compound was treated with CuCl in the presence of oxygen to accomplish deprotection and Glaser coupling in one pot to obtain 1,4-bis(2-thienyl)butadiyne in 72% yield. Many attempts were made to synthesize 1-phenyl-2,5-bis(2-thienyl)phosphole from 1,4-bis(2-thienyl)butadiyne by altering reaction conditions such as temperature, concentration, use of cosolvents, and use of microwave. All attempts resulted in either no reaction or formation of polymeric byproducts.
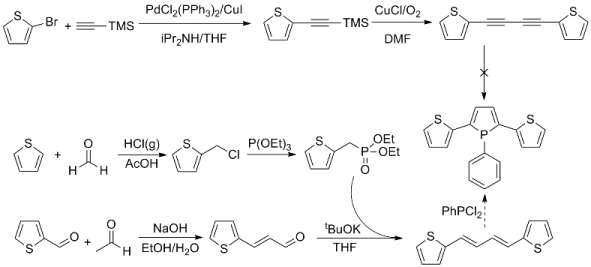
In search of an alternative synthetic route to 1-phenyl-2,5-bis(2-thienyl)phosphole, 1,4-bis(2-thienyl)butadiene has been chosen as a precursor instead of 1,4-bis(2-thienyl)butadiyne. It resulted in a more involved synthetic scheme. Treatment of thiophene with paraformaldehyde in the presence of HCl forms 2-(chloromethyl)thiophene in moderate yield. This compound is reacted with triethyl phosphite to give diethyl(2-thienylmethyl)phosphonate in 70-80% yield. Aldol condensation of 2-thiophenecarboxaldehyde with acetaldehyde gives 3-(2-thienyl)-2-propenal in 20-30% yield. Wittig type reaction with this aldehyde and diethyl(2-thienylmethyl)phosphonate gives the precursor 1,4-bis(2-thienyl)butadiene in 60-80% yield. A few attempts have been made to synthesize 1-phenyl-2,5-bis(2-thienyl)phosphole from 1,4-bis(2-thienyl)butadiene. Although the isolation of the compound is still in the process, 1H, COSY and 31P NMR suggests the presence of the target compound in the crude. Currently, our focus is on the optimization of the reaction conditions for each step and scaling up.
The Impact of the ACS PRF UNI Grant on the PI and Students
The UNI grant provided essential financial support for PI's research activities. The continuation of the research would have been impossible without UNI grant after the start up funding from the institution was exhausted for purchasing spectroscopic analytical instrumentations. Total of six undergraduate students were actively involved in the project during the semesters. One of the students was supported by this grant for the last summer. Another student who graduated in May with a BS degree decided to continue his research as a graduate student in the PI's group. Although he was not able to participate during the summer, he has been actively participating in the research activity since the beginning of the semester.
Plans for the Second Year
The priority is given to the synthesis of 1-phenyl-2,5-bis(2-thienyl)phosphole in large quantity and complete characterization of the compound utilizing spectroscopic and electrochemical techniques. The polymer of 1-phenyl-2,5-bis(2-thienyl)phosphole will be prepared and characterized for later comparison. 1-phenyl-2,5-bis(2-thienyl)phosphole will be treated with K2PtCl4 to obtain a platinum complex bearing chloride ligands and phosphole based ligands. This complex will be chemically polymerized via Hagihara coupling using chloride ligands on the platinum in the presence of diethynylbenzene. The resulting Pt-acetylide polymer will be spin-coated onto an ITO electrode and attempts will be made to cross link the polymer via electrochemical oxidation. The prepared 2D polymer matrix will be characterized by spectroscopic and electrochemical techniques. The preparation and characterization of 1-phenyl-2,5-bis(2-thienyl)phosphole should be accomplished in Fall 2011. Characterization of polymeric materials are to be done in Spring and Summer 2012.
