www.acsprf.org
Reports: DNI550427-DNI5: Tuning Dihydrogen Adsorption at Metal Centers of Surface-supported Supramolecular Networks
Steven L. Tait, PhD , Indiana University
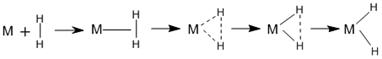
We utilize coordinatively-unsaturated metal centers in metal—organic frameworks at surfaces to study the tuning of these metal sites for di-hydrogen adsorption. The delicate challenge of binding the intact di-hydrogen molecule requires stable adsorption at the metal site through donation of σ electrons from H2 to unoccupied d orbitals of the metal, while avoiding excessive back donation (BD) to the H2 anti-bonding orbital. This balance determines the difference between molecular H2 adsorption vs. H—H scission and hydride formation (see Figure 1). Tuning is accomplished by substituting electron donating or withdrawing groups on the ligands.
Figure 1. Metal di-hydrogen interaction at varying strengths. For weak back donation (left) the H2 molecule is weakly attached and remains intact. With increasing back donation (to the right), hydride formation occurs.
A key scientific theme in this work is the question of whether metal centers in metal—organic complexes at surfaces can be tuned to the same degree as in solvated molecular complexes. The realization in recent years of highly-ordered surface structures by supramolecular self-assembly, including metal—organic coordination bonding, has opened up many opportunities for efficient and designable surface architectures. Here, the metal centers are in contact with the surface, which will also play a role in the charge state of the metal. This may provide opportunity for further tuning of the active adsorption site.
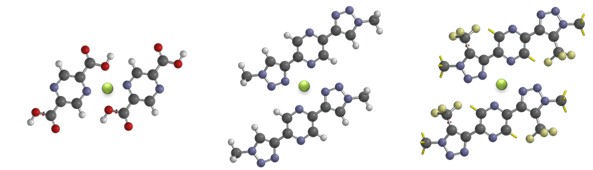
The system selected for study in this project is a complex of Cr atoms and pyrazine-2,5-dicarboxylic acid (PDA) (Scheme 1 left), on the Au(100) surface. Cr was selected because it transfers a significant amount of charge to Au surfaces upon adsorption. Thus, back donation from Cr to the anti-bonding orbital of the di-hydrogen is not expected to be severe, enabling labile di-hydrogen bonding. The compact geometry of this ligand will allow for a high packing density of Cr sites at the surface.
Consultation with collaborators with regard to the synthesis of ligands during this project has led to the design of the ligand shown in the center of Scheme 1, which offers advantages in a simple synthetic procedure, while still providing binding sites for Cr in a diverging geometry that will enable a high density of metal centers in a regular pattern on the surface. A key feature of this work is the addition of electron withdrawing or accepting groups to the ligands in order to tune the metal center and, consequently, impact the nature of di-hydrogen binding. At the right in Scheme 1 is an example of CF3 group substitution to steer the electronic state of the Cr sites.
Scheme 1.
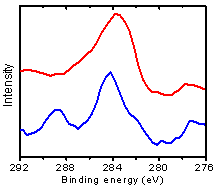
Organic and metal components are vapor deposited to a surface. The experiments are conducted under ultra-high vacuum conditions to provide clean and well-controlled experiments. We can control the surface coverage of each component in the system by the shutter open time of the evaporators. Studies in vacuum also allow the use of electron spectroscopy methods. For example, we have identified the adsorption of PDA using X-ray photoelectron spectroscopy (Figure 2).
Figure 2. X-ray photoelectron spectroscopy showing the C 1s core level peaks for the adsorption of PDA on a metal surface.
Di-hydrogen adsorption is accomplished here using a new pulsed supersonic molecular beam which can deposit the molecular hydrogen to the surface in a controlled way. Binding is observed by high-resolution electron energy loss spectroscopy and binding strength quantified by thermal desorption spectroscopy using a quadrupole mass spectrometer.
During the first year of support, we have been able to make important advances towards the goals of this project. Our pulsed supersonic molecular beam has been successfully assembled and is now operational. This required assembly and testing and integration of multiple beam components. We have successfully pulsed a gas beam to the surface using three differential pumping stages. The gas begins at a pressure above atmospheric pressure and is pulsed through a fine nozzle aperture using a fast solenoid valve. A skimmer removes most gas and allows for a collimated beam with a narrow energy distribution. As the beam passes through differential pumping stages, the background pressure is reduced so that the UHV analysis chamber pressure stays in the 10-9 Torr pressure range during beam operation. This molecular beam will allow us to pulse molecular hydrogen at the sample surface to probe hydrogen adsorption.
This project is providing an outstanding training opportunity for graduate student Amanda Lear. Ms. Lear has made enormous progress in bringing the pulsed supersonic molecular beam to the point where we can pulse gases to our surfaces through a computer controlled interface and record scattering data using a mass spectrometer. In addition to her work with the molecular beam and knowledge gained through literature searching and reading, Ms. Lear has developed skills in running preliminary experiments using Auger electron spectroscopy and high-resolution electron energy loss spectroscopy. Both of those tools will be critical in achieving the goals of the project. Amanda Lear has presented preliminary results at the Central Region meeting of the American Chemical Society (CERMACS) and also at the Turkey Run Analytical Chemistry Conference. Both of those meetings provide excellent opportunities for her to talk about her work and progress toward research goals, as well as to get advice, deeper understanding, and encouragement from peers and from more senior scientists. A second graduate student Jennifer Sandahl has recently joined our group and assisted in surface characterization of the adsorbed molecules. This project is providing graduate training opportunities for two women scientists.
This project is producing exciting results as we progress towards the research goals of realizing controlled di-hydrogen adsorption at surface-supported Cr sites and the ability to tune those sites through ligand design.