www.acsprf.org
Reports: ND1049126-ND10: Sustainable Synthesis of Nanoparticles
Charles Liotta, PhD , Georgia Institute of Technology
Charles A. Eckert, PhD , Georgia Institute of Technology
The goal of this project is to develop a synthesis method for producing monodisperse nanoparticles using reversible ionic liquids as switchable surfactants. In this method, the reversible ionic liquid can form a micelle around the gold salt. The gold salt can then be reduced and the reversible ionic liquid reversed to a non-ionic molecular liquid with heat, thus creating a switchable surfactant system as shown in Figure 1.
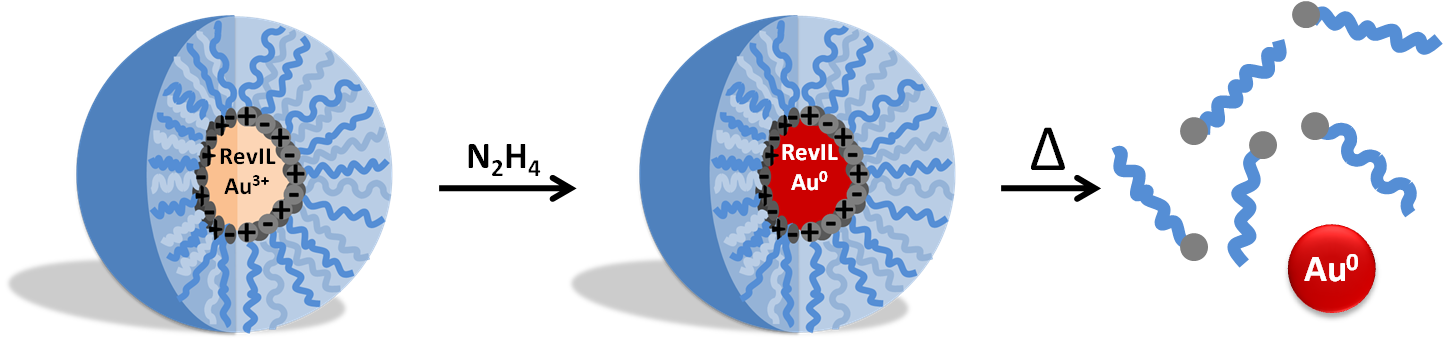
Figure 1 . Reverse micelle containing gold salt and RevIL; reduction of gold salt to form gold nanoparticles; destruction of micelles and release of nanoparticles.
Surfactants are commonly used in nanoparticle synthesis as capping agents to aid in the control of size and shape of the nanoparticle, which regulation of is highly important for catalytic activity. Removal of the traditional surfactant capping agents requires additional steps (e.g. washing, calcination, etc.), which can be both time-, energy- and waste-intensive.
Our research group has pioneered reversible ionic liquids (RevILs), which exhibit a drastic switch in properties upon reaction with CO2.1 We report here the use of one-component RevILs as switchable surfactants. Turning the ionic form of the capping agent "on" allows us to use reverse micelles as synthetic templates for nanoparticles; turning the capping agent "off" allows us to easily separate the nanoparticles from solution.
Trialkylsilylpropylamines react with carbon dioxide to form a carbamate anion and an ammonium cation, as seen in Figure 2.2
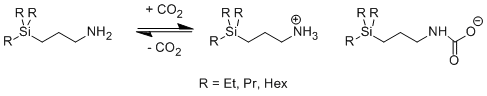
Figure 2 . One-component RevILs.
Heating or sparging with an inert gas drives off the CO2 and regenerates the molecular precursors—this process can be repeated numerous times.1 Our previous work on this topic used an earlier generation of RevILs (two-component RevILs), which required equimolar mixtures of a guanidine and a straight-chain alcohol to react with CO2, forming a guanidinium cation and an alkylcarbamate anion. Using the one-component RevILs eliminates the need for precise equimolar amounts of molecular precursors. The trialkylsilylpropylamines are not commercially available, but are easily synthesized in house via a platinum-catalyzed hydrosilylation reaction, seen in Figure 3.
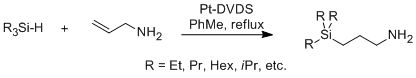
Figure 3 . Synthesis of trialkylsilylpropylamines. Reaction time varies based on silane substituents.
Our group has synthesized a number of these silylated amines; the structures and abbreviations of some selected compounds are listed in Table 1.
Table 1 . Abbreviations for various trialkylsilylpropylamines.
It has been shown that the size and shape of nanoparticles formed using reverse micellar templates can be varied by controlling the weight ratio of the gold salt to the ionic liquid (WHAuCl4/WRevIL).3 We therefore hope to control the nanoparticle size by systematically varying the proportions of gold salt, RevIL, and continuous phase in our system. Experimental
All chemicals were purchased from Sigma-Aldrich and used as received without further purification. For the 1-component RevILs, the trialkylsilylpropylamines were synthesized via a platinum-catalyzed hydrosilylation reaction. To a solution of silane (1 equivalent) in anhydrous toluene under argon was added 0.2 mol% Pt-DVDS in xylenes. Two equivalents of allyl amine was added, and the reaction mixture was heated at 110 °C. The reaction time varied based on the steric bulk of the silane substituents. Upon completion, the solvent was removed under reduced pressure and the crude product distilled to yield a clear oil. The structures of the products were characterized via 1H and 13C NMR, as were the structures of the RevILs formed.
A typical nanoparticle synthesis was carried out as follows: The RevIL was formed by sparging CO2 through a trialkylsilylpropylamine for 75 minutes at 200 mL·min-1. HAuCl4 was then dissolved in the RevIL and mixed with hexane to form reverse micelles. A solution of hydrazine in THF was used for reduction. Following reduction, reversal of the switchable surfactant was achieved by heating in an oil bath for approximately one hour. Nanoparticles thus formed were analyzed using an HP8453 UV-Vis spectrophotometer and a Hitachi HF-2000 FEG transmission electron microscope. Results and Discussion
After successfully showing the formation, dissolution, and reformation of the reverse micelle system with methyl orange and two-component RevILs, we investigated the formation of gold nanoparticles using our one-component RevILs.
We have successfully formed, deposited, and imaged unsupported gold nanoparticles using both TPSA and THSA. Preliminary experiments were carried out using THSA, as its structure was deemed to be most similar to that of a surfactant. With a WHAuCl4/WRevIL of 7.789 x 10-3, we were able to obtain nanoparticles with an approximate diameter of 15-20 nm, as seen in Figure 4.
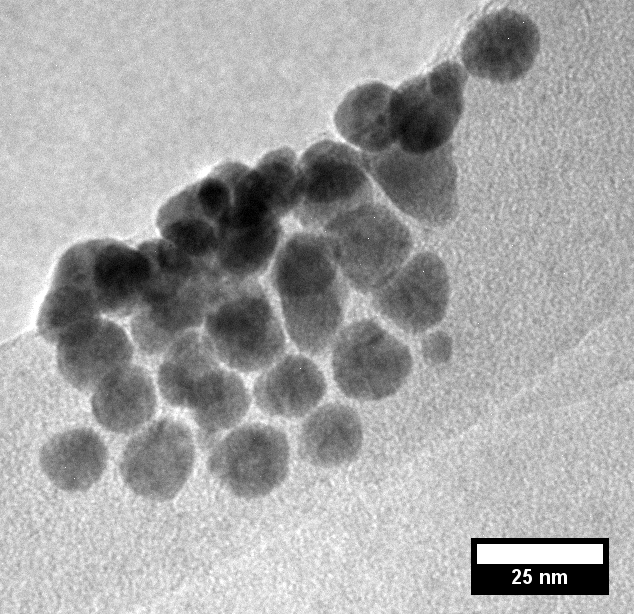
Figure 4 . Gold nanoparticles averaging between 15 to 20 nm in diameter, formed using THSA-IL.
However, these nanoparticles are not monodisperse.
After a series of modifications to the experimental procedures including adjustment of reducing agent solution and introduction method, RevIL and reaction conditions, much smaller monodisperse nanoparticles were formed. Using TPSA as our RevIL, at a WHAuCl4/WRevIL of 7.997 x 10-3 comparable to the above image, we were able to obtain nanoparticles with an average diameter of 6.1nm and a standard deviation of 0.9nm, shown in Figure 5.
Figure 5 . Gold nanoparticles synthesized using TPSA-IL. (Top) TEM image. (Bottom) Size distribution chart.
Optimization of the deposition method of these gold nanoparticles onto oxide supports for further use as catalysts is ongoing.
Works cited
- Hart, R.; Pollet, P.; Hahne, D. J.; John, E.; Llopis-Mestre, V.; Blasucci, V.; Huttenhower, H.; Leitner, W.; Eckert, C. A.; Liotta, C. L., Benign coupling of reactions and separations with reversible ionic liquids. Tetrahedron 2010, 66 (5), 1082-1090.
- Blasucci, V.; Dilek, C.; Huttenhower, H.; John, E.; Llopis-Mestre, V.; Pollet, P.; Eckert, C. A.; Liotta, C. L., One-component, switchable ionic liquids derived from siloxylated amines. Chemical Communications 2009, (1), 116-118.
- Liu, J. H.; Cheng, S. Q.; Zhang, J. L.; Feng, X. Y.; Fu, X. G.; Han, B. X., Reverse micelles in carbon dioxide with ionic-liquid domains. Angewandte Chemie-International Edition 2007, 46 (18), 3313-3315.
