www.acsprf.org
Reports: DNI150350-DNI1: Regioselective Catalytic C-H Oxidation of Hydrocarbons in Aqueous Solution
Richard Hooley, PhD , University of California (Riverside)
Narrative Report Form - Grant 50350-DNI1
Our work in this grant period has focused on the synthesis of molecules that are capable of both molecular recognition and reactivity, in order to provide regioselective oxidation of simple hydrocarbons based on proximity effects. Regioselective catalytic CH oxidation is extremely rare in the literature. The catalyst not only requires good reactivity, but a recognition element must be incorporated into the catalyst to discriminate between electronically identical reactive sites. We seek to use proximity-directed reactivity to control oxidation selectivity and create catalysts that are truly biomimetic.
In this grant period, we have studied the synthesis and properties of two possible targets for this process: derivatized resorcinarene-based hosts (cavitands) and tren-based macrocyclic cages. To create a catalyst that is capable of regioselective CH oxidation, three factors must be addressed: the catalysts must be capable of non-covalently binding the target molecule and orienting it in one direction, must incorporate a catalytic motif (usually a high-oxidation state metal species) and must allow release of the product from the binding site. The molecular recognition elements consist of a defined cavity for non-covalent shape-based recognition, which is either open-ended (cavitands) or fully closed (tren cages). Multiple metals can be coordinated to appended triazole, pyridine or tren-type nitrogen ligands for catalytic activity. To address the issue of catalysis (and minimize product inhibition, a well-known issue in supramolecular catalysis), we focused on performing the reactions in water - by exploiting hydrophobic control of guest binding, turnover of the more hydrophilic oxidation products is possible. This, of course, requires synthesis of catalysts that are both tolerant to, and soluble in aqueous solution.
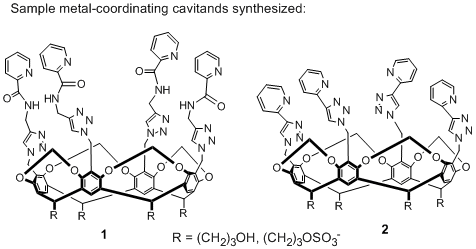
Our initial target is the open-ended cavitand structures shown in Figure 1. We have synthesized a series of water-soluble cavitands with appended metal-coordinated binding sites. In order to confer water-solubility on the complexes, the base (or feet) of the cavitands are alcohol groups that have been converted to phosphate or sulfate groups for greater water solubility. Incorporation of metal-coordinating ligands to the cavitand rim was achieved by CuAAC chemistry. The resulting substituted triazoles at the cavitand rim can coordinate metal species such as Fe2+ and Cu+. Because the species contain multiple metal binding sites, different conformations are possible. Different hosts have been synthesized that are capable of binding two metal ions (e.g. 1 binds two copper (I) ions, 2 binds two Fe(II) ions) at the sides of the receptor, retaining the defined cavity. Characterization of the properties of these molecules has been performed by NMR spectroscopy, MALDI mass spectroscopy and X-ray crystallography. Preliminary results show that these metal-binding species are capable of simple olefin oxidations in aqueous solution, and we are investigating their host properties and oxidation efficiency in aqueous hydrogen peroxide solution. Our work on the synthesis, metal binding properties and reactivity of these cavitands will be submitted for publication shortly. Future work will be focused on optimizing the oxidation reaction and extending this work to regioselective oxidation of saturated hydrocarbons.
Figure 1. Two examples of target hosts synthesized.
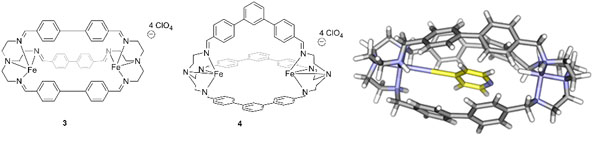
To provide a more rigid binding environment than in the cavitands described above, we have studied the synthesis of tren-based macrocycles (Figure 2). The tren unit is a well-precedented ligand that can coordinate metal ions and has been used previously in oxidation catalysis. By incorporating this unit in an enclosed cage, a defined cavity can be created and only suitably sized bound substrates are exposed to the catalytic site. We have synthesized two different tren-based cages and shown their ability to coordinate multiple, high-valent metals such as Fe3+ on the interior of the cavity. These cages are capable of binding small species such as 4-cyanopyridine inside the cavity of the macrocycle. Future work on these scaffolds will focus on using this system in aqueous media for oxidation reactions of hydrocarbons.
Figure 2. Metal-containing macrocycles synthesized, including molecular visualization of cage 3
Also in this grant period, we have published two journal articles on work partially supported by this grant. These publications involve study of the formation of nanoscale cage structures that assemble through reversible metal-ligand interactions and display functionality on the interior of the cavity. These molecules are bis-pyridyl M2L4 paddle-wheel structures, and we have analyzed their structure, ligand dynamics and ligand exchange behavior by NMR, X-Ray diffraction and UV-Vis methods. We have shown the first example of self-sorting behavior through solely steric considerations by altering the functionality on the interior of the complex. These studies give knowledge of the effects of reactive functionality and molecular recognition on self-assembled systems.
This research has had a strongly beneficial impact on my graduate students (and their undergraduate co-workers). New techniques learned by my students include a variety of processes in synthetic organic chemistry, including organometallic coupling reactions as well as more classical organic chemistry. Analysis of these species involves a number of techniques such as cyclic voltammetry, dynamic and 2D NMR methods, mass spectrometry and X-Ray crystallography, most of which were unknown or unfamiliar to my students at the start of the year.