www.acsprf.org
Reports: UR449182-UR4: New Directions in Understanding Conformational Preferences of 1,2-Disubstituted Ethanes
John D. Roberts , California Institute of Technology
This research extends the conformational study of trans-1,2- and cis-1,3-cyclohexanedicarboxylic acids discussed in a PRF Report of February 2011. The essence earlier concerned conformations of trans-1,2- and cis-1,3-cyclohexanedicarboxylic acids normally expected to exist in cyclohexane chair forms with the both carboxyl (X) groups in the trans-diaxial positions 1a or trans-diequatorial as 1b, and cis-diaxial 2a or cis-diequatorial 2b, where the other lettered positions represent particular hydrogens. Because sizable substituents are substantially more stable in equatorial, rather than axial positions, those familiar with cyclohexane ring conformations will conclude, and our experimental NMR proton-proton coupling data for water and DMSO as solvents confirm, that conformer 1b substantially more stable than 1a and 2b is more stable than 2a.1 This holds for both acids, also for their monoanion and dianion salts.
This predominance of equatorial forms (80-100%) effects interactions between the carboxyl groups for the trans-1,2- and cis-13- diacids, especially for intramolecular hydrogen bonding. Useful diagnostic tests for such bonding include; 1) Are the acid groups situated for an intramolecular hydrogen bond to be formed at all? ; Yes, 1b and 2a. No, 1a and 2b; 2) Are the relative acidities of the acids correct? Much work suggests intramolecular hydrogen bonding requires diacids with their K1 ionizations large to be good proton donors and K2 ionizations small to hang tightly to adjacent anionic acceptor groups. Is K1 /K2 in water > ~104; 3) NMR chemical shift of the hydrogen-bonded proton downfield, by 14-20 ppm? 4) Are vicinal proton couplings in the connecting group reasonable?
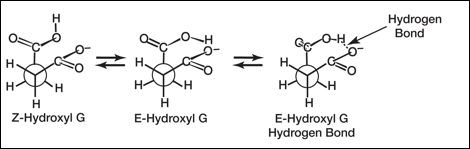
Succinic acid in water provides testing of these diagnostics, with K1 /K2 about 22 and, while succinate monoanion looks to have the proper structural features, an intramolecular hydrogen bond is absent in water, failing diagnostics above, except 1) and 4). The hydrogen-bond forming sequence for monosuccinate ion (below), starts with the carboxyl OH hydrogen in the stereochemically more stable Z (anti) position (useless for H-bonding) to the ~5 kcal less stable E (syn) O-H bond (favorable for H-bonding). However, water competes here with hydrogen-bonding by solvating the negative carboxylate so much that it loses all interest in stabilization by the carboxyl group.
However, in DMSO, succinic acid has K1 /K2 = 1.6 x 107, while the trans-1,2 and cis-1,3- acids are 4 x 106 and 6.3 x 102, respectively. Thus, succinate monoanion and 1b are candidates for intramolecular bonding in DMSO, but not 2a. DMSO is much better than water for fostering formation of hydrogen bonds because, unlike water, DMSO is a poor solvator of anions. Not only is Ki /K2 small for 2a, hydrogen bonding has to overcome the favorability of diequatorial-2b over diaxial-2a to get the acid groups close enough together. Clearly, cis-12 acid is a dud for interactions between its carboxyls. The cure is an analog, where the 2a structure would comparably stable to 2b.
This goal arrived by synthesizing trans-5-tert-butyl-cis-1,3- diacid, where a large group R (5-tert-butyl) is placed at the g positions of 2a and 2b. Being bulky, 5-equatorial R stabilizes R-2a, but when axial, 5-R destabilizes R-2b, and the behaviors change dramatically, Figure 1.
Figure 1. Bar-graph of conformational preferences of 5-tert-butyl-cis-1,3 diacid ionization states. Red = aa, blue = ee, and green = tb.
Now, K1 /K2 = 1 x 106, the proton of the hydrogen-bond at equivalence pH for the monoanion has a chemical shift of 19.35 ppm at -80°C, as expected for a hydrogen-bonded proton, and also is in the aa conformation as evidenced from the proton-a with proton-c couplings of R-2a. Equilibrium between R-2a and R-2b is not one-sided in favor of aa, but while monoionized R-2a readily forms the unusual H-bond, the corresponding monomethyl ester is more stable as eeZ.
To our knowledge, this is the first example of formation and isolation of an intramolecularly hydrogen-bonded diacid of the general type, X-CRR'-CRR'-CRR'-X, where the X's are carboxyl groups and the Rs are not specified.