www.acsprf.org
Reports: DNI149533-DNI1: Reaction Design for Nucleophilic Fluorination with Chiral Lewis Base Catalysts
Abigail G. Doyle, PhD , Princeton University
Fluorine-containing organic molecules serve as critical pharmaceuticals, agrochemicals, performance materials, and radiotracers for imaging processes. Unfortunately, the availability of methods for the construction of C–F bonds severely limits the discovery and synthesis of these important targets. Particularly challenging in this context is the development of methods for the mild and selective incorporation of nucleophilic fluoride, the naturally occurring and least expensive form of fluorine, into organic targets. Use of anhydrous HF is plagued by difficulties with its safety and handling, metal fluorides suffer from sparing solubility in organic solvents, and reagents with enhanced solubility like tetraalkylammonium fluorides display high Brznsted basicity.
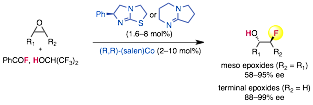
Last year, we reported a highly enantioselective method for fluoride ring opening of meso and terminal epoxides by the use of commercial benzoyl fluoride as a bench-stable, organic-soluble latent fluoride source. Notably, the cooperative catalytic fluorinations proceeded at room temperature in standard glassware and did not require exclusion of moisture or air. A consequence of these mild conditions was that substrates containing traditionally fluoride-labile substituents such as silyl ethers are stable to the reaction conditions and undergo C–F bond formation with good reaction efficiency. This year, to demonstrate the practicality of this reaction, we prepared an Organic Synthesis article describing the reaction of styrene oxide on 10g scale.
Over the past year, we have engaged in mechanistic studies of the fluorination reaction to address the following: (1) the origin of the matched-mismatched effect on rate and enantioselectivity, which had little precedence in Lewis acid-amine cocatalysis; (2) the role of the amine cocatalyst; and (3) the low reaction rates, which limited the synthetic applicability of the method. We conducted kinetic, spectroscopic, non-linear, kinetic isotope effect, and Hammett studies, which allowed us to propose a mechanism involving a (salen)CoFHF as the active nucleophile and a role for the amine co-catalyst as a ligand for cobalt.
These studies led us to prepare and evaluate a tethered cobalt catalyst for the fluorinations, which afforded significantly enhanced rates (in some cases < 5 minute reaction times) and enantioselectivities for epoxide ring opening. A current direction of our research is to pursue applications of this catalytic method toward the rapid radiosynthesis of 18F-labelled molecular imaging agents for neurodegenerative diseases.
We anticipated that the reactivity of the chiral, organic-soluble transition metal bifluoride could be expanded to other electrophilic reaction partners, such as aziridines in the synthesis of beta-fluoro amines. Under the support of the ACS PRF, we have begun to investigate cooperative fluoride ring-opening reactions of aziridines and have obtained promising preliminary data to suggest that such a route will be feasible. Present in >150 drug candidates in phase II and III clinical trials, eta-fluoro amines are perhaps the most important fluorine-containing target for new reaction discovery. Successful development of this method would streamline the synthesis of known therapeutic agents and enable the preparation of previously inaccessible bioactive small molecules.
In summary, we have demonstrated that mild, operationally convenient, and selective methods for C–F bond formation are possible by a nucleophilic fluorination approach. Key to the development of these methods was our identification of a novel fluoride source for C–F bond formation and the use of an inexpensive transition metal catalyst. The Doctoral New Investigator grant from the ACS PRF has been instrumental in jump-starting this program and we are extremely grateful for the support.