www.acsprf.org
Reports: ND149109-ND1: Alkali and Alkaline Earth Metal Complexes for Catalytic Hydroaminations
Kai C. Hultzsch, PhD , Rutgers, the State University of New Jersey
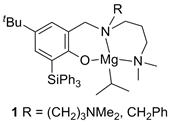
The application of alkaline-earth metal complexes as substitutes for transition metal catalysts in alkene hydrofunctionalizations has drawn increasing attention in recent years due to their abundance, biocompatibility and chemical behavior resembling that of the rare-earth elements. Of particular interest is the so-called hydroamination reaction of alkenes, in which an amine N-H bond adds to an unsaturated carbon-carbon bond. This process offers a waste-free, highly atom-efficient and green pathway to produce nitrogen-containing compounds, such as amines, enamines and imines, which are valuable and industrially important bulk chemicals, specialty chemicals and pharmaceuticals. While most research has focused on the development of transition metal-based catalyst systems, the aim of our project is to develop efficient alkali and alkaline-earth metal-based catalysts. Unfortunately, alkaline-earth metal complexes are prone to facile Schlenk-type ligand redistributions that can result in catalyst deactivation and hamper efforts to perform these transformations in a stereoselective manner due to the formation of achiral catalytic active species. An important initial goal was therefore to develop a catalyst system that can resist such ligand exchange processes. During the previous funding period Dr. Xiaoming Zhang studied the catalytic activity of the achiral phenoxyamine magnesium complexes 1. Based on these promising results, Dr. Zhang proceeded to develop a chiral variant of this system.
Chart 1. Previously studied achiral triphenylsilyl-substituted phenoxyamine magnesium complexes.
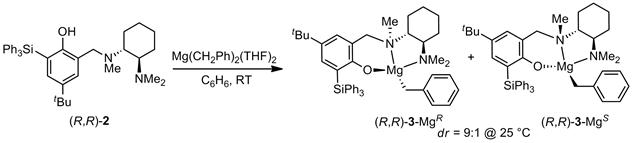
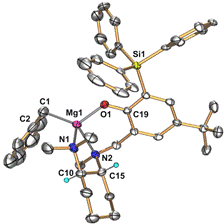
For this purpose, we decided to utilize the chiral phenoxyamine ligand (R,R)-2 in which the increased steric demand of the triphenylsilyl substituent should eliminate undesired ligand exchange processes and improve stereoselectivity. Reaction of (R,R)-2 with Mg(CH2Ph)2(THF)2 produced the phenoxyamine magnesium complex (R,R)-3 as a 9:1 diastereomeric mixture (Scheme 1; the two diastereomers differ in the chirality at magnesium and the central N-methyl amine group). The (R,R)-3-MgR diastereomer was also structurally characterized (Figure 1).
Scheme 1. Synthesis of the chiral phenoxyamine magnesium complex (R,R)-3.
Figure 1. ORTEP diagram of the molecular structure of the chiral phenoxyamine magnesium complex (R,R)-3-MgR.
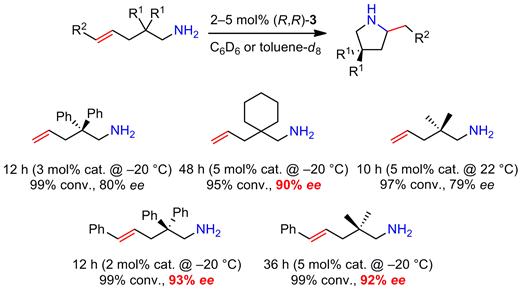
Complex 3 was shown to be a significantly more potent catalyst in the cyclization of aminoalkenes (Scheme 2) than the previously studied complexes 1. The cyclization of various aminopentenes proceeded smoothly at temperatures as low as −20 °C with enantioselectivities as high as 93% ee. These catalytic results are remarkable for several reasons. First, this catalyst is the first alkaline-earth metal catalyst that operates at such low temperatures (note: this is despite the fact that calcium catalysts are in general more active than magnesium) and in general only very few transition metal catalysts are known to be active under these conditions. Second, the enantioselectivities are far superior to any previously asymmetric hydroamination catalyzed by an alkaline-earth metal catalyst (the highest enantioselectivity of 36% ee was reported in 2011). The results of this study have been communicated in a VIP paper in Angewandte Chemie.
Scheme 2. Catalytic asymmetric hydroamination/cyclization of aminoalkenes using the chiral phenoxyamine magnesium catalyst (R,R)-3.
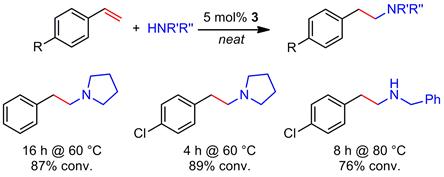
In order to document the high catalytic activity of the new catalyst system 3, we also investigated the addition of amines to vinyl arenes. The reaction proceeds with high anti-Markovnikov selectivity (Scheme 3). Catalyst 3 is the first magnesium-based catalyst to achieve this transformation.
Scheme 3. First magnesium-catalyzed intermolecular hydroamination of vinyl arenes with amines.
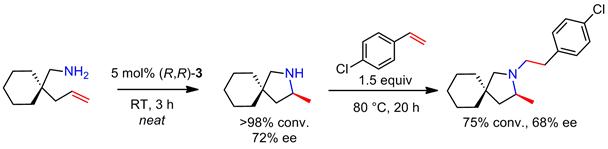
The competency of this magnesium catalyst was also demonstrated in an intramolecular/intermolecular tandem hydroamination reaction (Scheme 4).
Scheme 4. Magnesium-catalyzed tandem intramolecular/intermolecular hydroamination.
Current investigations focus on the development of corresponding calcium complexes that promise to exhibit even greater catalytic performance.