www.acsprf.org
Reports: UR149493-UR1: Development of Enantioselective Nitrogen Arylation Reactions
Russell D. Viirre, PhD , Ryerson University
The development of new reactionmethodologies capable of enantioselectively forming chiral products fromachiral starting materials is an area of intense research. When the selectivity in such reactionsis induced from only a very small amount of chiral catalyst, the methodologycan be a cost-effective way to form very valuable chiral products fromrelatively inexpensive starting materials. With this goal in mind, we have been exploring the utilityof the Buchwald-Hartwig reaction as an enantioselective reaction.
In general, a Buchwald-Hartwigreaction is a palladium-catalyzed cross-coupling reaction between a nitrogennucleophile, and an aryl halide, forming an aromatic C-N bond. While the literature contains hundredsreferences to this important reaction, fewer than ten studies have explored thepossibility of using a Buchwald-Hartwig reaction to induce asymmetry in aproduct, and most of these achieved only modest selectivities.
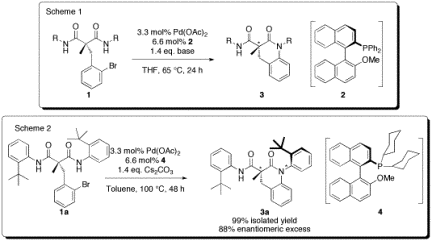
In the first year of this ACS PRFUndergraduate grant, we developed and optimized the enantioselectivering-closing Buchwald-Hartwig reaction of symmetrical malonamides 1 (Scheme 1). Under the optimized conditions, employing the chiral phosphine ligand (R)-MOP(2), the cyclized products 3 are obtained in excellent yields and up to 96%ee. In the second year of thisgrant (the subject of this current report), we have developed a synthesis forthe related dicyclohexylphosphine ligand 4, and shown that it is the ideal supporting ligand for the asymmetricintramolecular Buchwald-Hartwig reaction of 2a (Scheme 2). This formation of 3a isnotable since the product contains both a chiral center, as well as a chiralaxis (due to blocked rotation about the nitrogen to o-t-butylphenylbond). In this case, from achiralstarting material 2a, only a singlediastereomer is formed in 99% yield and 88% ee. The configuration of the major enantiomer was confirmed byX-ray crystallography. In additionto these results, we have made exciting headway into extending our methodologyto the synthesis of larger and smaller rings, as well as intermoleculardesymmetrization reactions, and the development of new catalysts. All of this work has been carried outby students at the undergraduate and Masters level.