www.acsprf.org
Reports: B447629-B4: Pentacyclo[4.3.0.0(2,4).0(3,8).0(5,7)]non-4-ene: Synthesis, Reactivity, Matrix Isolation Spectroscopy, Calculations, and Physical Study of Reaction Products
Mark A. Forman , Saint Joseph's University
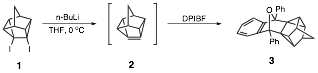
Undergraduate students in my research group have shown that treatment of 4,5-diiodopentacyclo[4.3.0.02,4.03,8.05,7]nonane (1) with n-butyllithium or t-butyllithium at 0 oC in the presence of a trapping agent such as 1,3-diphenylisobenzofuran (DPIBF) furnishes a 25-30% yield of the "trapped" product 3, thus providing strong evidence for the formation of 2.
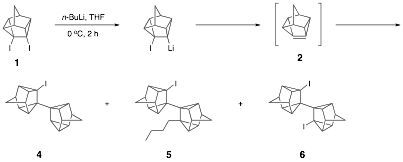
Dehalogenation of diiodide 1 with n-butyllithium in the absence of 1,3-diphenylisobenzofuran or other trapping agents affords alkyllithium addition products 4, 5, and 6, along with smaller amounts of other alkyllithium addition products. These three products were also present in the aforementioned Diels-Alder trapping reaction of 1 with n-butyllithium and DPIBF, albeit in smaller quantities. Alkyllithium addition products are also formed when the dehalogenation is conducted with t-butyllithium, although obviously structure 5 is not formed. It is well known that alkyllithiums add readily to highly pyramidalized alkenes (see Tetrahedron 2005, 61, 5147), and in fact, isolation of these addition products provides further evidence for the formation of pyramidalized alkene 1.
In order to further study the chemistry and reactivity of 2, an alternative route that does not utilize alkyllithiums to generate the pyramidalized double bond must be developed. Thus, one focus of our research during the grant period has been the synthesis of these alternative precursors. An additional focus has been the use of diiodide 1 to generate 2 without using alkyllithiums.
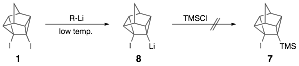
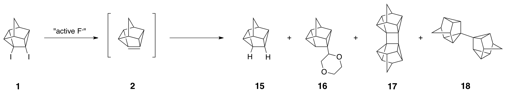
In his synthesis of cubene, Eaton observed that alkyllithiums reacted rapidly with the pyramidalized double bond (see J. Am. Chem. Soc. 1988, 110, 7230-7232). The high reactivity of cubene with alkyllithiums in the reaction mixture prevented an exploration of the reactivity of the cubene double bond with reagents other than alkyllithiums, a situation similar to what is observed in our synthesis of 2. Thus Eaton developed another route to cubene without using alkyllithiums via the fluoride ion induced elimination of 1-halo-2-(trimethylsilyl)cubanes. In a similar manner, 4-iodo-5-(trimethylsilyl)pentacyclo[4.3.0.02,4.03,8.05,7]nonane (7) offers an alternative route to 2 via treatment with active fluoride ion.
Previously, attempts to synthesize 7 via lithiation of diiodide 1 at low temperature followed by addition of chlorotrimethylsilane (TMS-Cl), were unsuccessful, as lithium iodide elimination from 8 was rapid even at low temperature; trapping with TMS-Cl could not compete with elimination.
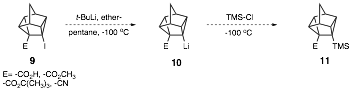
Moreover, attempted lithiation of molecules of type 9 (E=-CO2CH3 ,-CO2H, -CO2C(CH3)3, -CN), and subsequent trapping with chlorotrimethylsilane also did not lead to molecules of the type 11 (primarily) due to akyllithium addition to the electrophilic E groups.
To avoid competition from addition of alkyllthiums to the carbonyl and cyano groups present in 9, the t-butylester 13 was synthesized, as it is relatively unreactive toward alkyllithium addition to the hindered ester carbonyl. Currently, we are investigating metalation of t-butylester 13 with t-butyllithium followed by chlorotrimethylsilane quench to yield 14. Hydrolysis and Barton iododecarboxylation should lead to 7. Previously we had synthesized the diisopropyl amide derivative, we encountered problems converting the unreactive diisopropylamide to the carboxylic acid needed for decarboxylative iodination.
An alternative approach to 2 not utilizing alkyllithums involves the dehalogenation of 1 with reactive metals such as sodium and potassium and alloys such as sodium amalgam. Other groups have utilized this method extensively for the synthesis of pyramidalized alkenes. Thus, reaction of 4,5-diiodopentacyclo[4.3.0.02,4.03,8.05,7]nonane (1) with excess molten sodium in refluxing 1,4-dioxane gave a mixture of four main products by GC–MS. The product eluting first corresponded to the known reduction product 15; the second product is likely 16 (we have only crude NMR and GCMS at this point), derived from the addition of the intermediate pyramidalized alkene and the solvent (1,4-dioxane). This product is formed in very small quantities and, to this point, has been exceedingly difficult to isolate. The third product has been identified by single crystal x-ray analysis as cyclobutane dimer 17. The fourth major product has recently been identified as dimer 18.
As a result of the support from this ACS-PRF grant, a total of ten students have participated in this project. Thus far, six of these students have presented posters at American Chemical Society (ACS) National Meetings. Additionally, all of these students have also presented at local and regional meetings such as the ACS Philadelphia Section Poster Day and the Philadelphia Organic Chemists Club Poster Day.
The research described in this proposal will continue to provide a current, in-depth and diverse research experience to my students. Over the past twelve years, I have mentored twenty-eight full-time undergraduate research students at Saint Joseph's University. These students have gone on to seek advanced degrees in areas such as organic chemistry, physical chemistry, inorganic chemistry, biochemistry, and molecular biology. Other students currently seek and have completed medical degrees and other professional degrees.
The funding of this project has allowed all of the research students to be exposed to a variety of modern organic synthetic techniques, and to routinely run reactions under an inert atmosphere using Schlenk procedures and in an inert atmosphere glove bag. The nature of this work will provide my research students with not only a strong experience in synthetic organic chemistry, but also an introduction to a broad range of spectroscopic methods, including NMR, FTIR, and MS, and matrix isolation spectroscopy.