www.acsprf.org
Reports: UNI549454-UNI5: Adsorption of Thiophenes at Liquid/Solid Interfaces
Katherine E. Plass, PhD , Franklin and Marshall College
The organization of molecules at solid interfaces can have a profound influence on interfacial properties and affect lubrication, catalysis, and electron transfer processes. Thiophenes are common in organic semiconductors, which are incorporated into devices with interface-dependent behavior. Thus, understanding the self-assembly of these species at solid interfaces is thus of great interest. We investigate the effect of thiophene groups on self-assembly, thereby distinguishing the behavior of thiophene-containing species from that of the more-studied hydrocarbons. We have focused on observing the self-assembly behavior of a series simple alkyl-decorated thiophenes (Figure 1) using scanning tunneling microscopy (STM). Comparison of the patterns that these structurally related species form at the liquid-graphite interface provides insight into the competition between various intermolecular and substrate-molecule interactions.
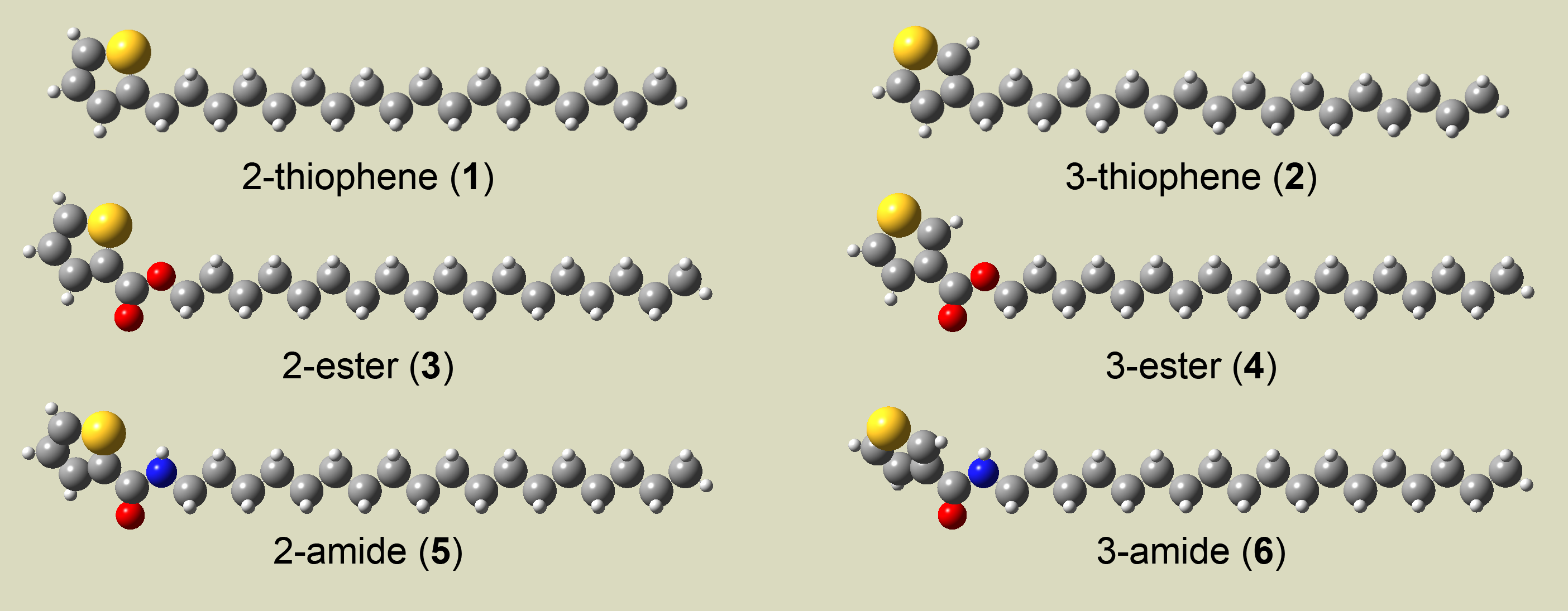
Figure 1. The structurally related alkyl-decorated thiophene species whose self-assembly will be observed at the liquid-solid interface. These species differ in the substitution position on the ring (1, 3, and 5 are substituted at the 2-position while 2, 4, and 6 are substituted at the 3-position) and in the attachment chemistry (1 versus 2 and 3 versus 4).
The series of thiophenes selected for investigation are shown in Figure 1. Each molecule contains an octadecyl chain attached to the thiophene either by a simple C-C bond (1 and 2), an ester-linkage (3 and 4), or an amide-linkage (5 and 6). The alkyl chain is bound either in the 2-(1, 3, and 5) or the 3-(2, 4, and 6) position of the thiophene ring. Recent syntheses have been carried out in collaboration with Prof. Kristopher Kolonko of Franklin & Marshall College. STM images of several of these molecules are compared below.
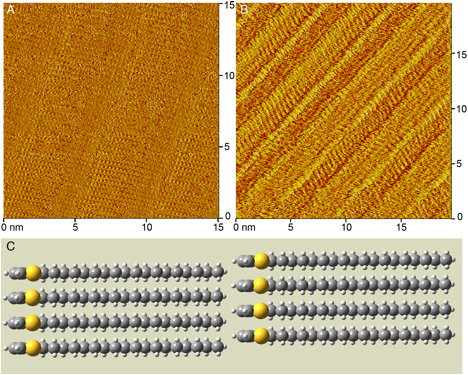
Figure 2. STM images of the self-assembled monolayers formed at the phenyloctane-graphite interface by species 1 (a) and 3 (b). These are current images collected in quasi-constant height mode. The bright yellow areas denote increased tunneling current. A computational model of 1 (c) is provided that shows the head-to-tail configuration, ring alignment, and perpendicular orientation of the alkyl chains observed in the STM image.
Comparison between the monolayers of 1 and 3 (Figure 2a,b) shows a distinct difference in the self-assembly of these two structurally similar species due to the introduction of an ester functionality (3) versus a simple alkyl chain (1). 1 self-assembles into lamellae in which the alkyl chains are at 90° angles to the propagation direction (Figure 2c). The spacing between columns (2.7 ± 0.8 nm) coincides with the length of one molecules. This suggests that the molecules in adjacent columns are arranged in a head-to-tail configuration. The alignment of the small bright spots that represent hydrogen atoms indicates that the carbon backbone is perpendicular to the substrate, an unusual orientation for alkyl chains on HOPG. This packing would optimize neither the interactions between neighboring alkyl chains nor interactions with the graphite substrate. Two factors would favor this packing, however. Packing density is maximized in this configuration, as are the p-p interactions thiophene rings. While the packing behavior of 3 (Figure 2b) is still being modeled, the STM image (Figure 2b) shows very distinct packing in comparison to 1 (Figure 2a). Notably, there is an angle between the alkyl chains and the propagation direction. A difference in the contrast between adjacent columns may suggest an alternation in the arrangement of the ester-containing species visible either due to the ester functionality or the asymmetry of the ring.
Figure 3. STM images of the self-assembled monolayers formed at the phenyloctane-graphite interface by species 5 (a) and 6 (b). Tentative models of the packing in both polymorphs of 5 (c,d) and the dominant pattern of 6 (e) are shown.
Comparison of the STM images of molecules with amides attached in the 2- versus the 3-position (5 versus 6) illustrates the effect of geometry on self-assembly. First, the amide attached at the 2-position (5) demonstrates polymorphism. Figure 3a shows two distinct packing patterns. The dominant pattern forms bright, unbroken columns of thiophene rings. These bright columns are spaced 2.6 ± 0.3 nm apart, suggesting a head-to-tail packing (Figure 3c). The tilt between the thiophene ring and the alkyl chain is suggested both by molecular modeling and the image. Molecular modeling confirms that this molecular conformation is a local energy minimum. The STM image shows both bright and dim columns in the upper domain. This could be explained by a difference in the orientation of the thiophene rings with respect to the tip. An additional packing pattern is apparent in the lower right-hand corner of the image (Figure 2a). Here, molecules assemble such that the columns of thiophene rings are broken periodically. Every 2.3 ± 0.1 nm (approximately 6 molecule widths), the thiophene rings move 1.2 ± 0.1 nm to the right. This periodic movement may help relieve strain that builds up in the monolayer. The 3-substituted isomer (6) exhibits unbroken columns of aligned thiophene rings, but they are spaced further apart than those for 5 (4.7 ± 0.3 nm versus 2.6 ± 0.3 nm). The spacing is nearly doubled, and coincided with the length of two molecules, suggesting a head-to-head arrangement (Figure 3e). A S-S intermolecular interaction makes the head-to-head configuration more favorable than the head-to-tail configuration for the 2-amide. Such a S-S interaction is possible for 6, because the S atoms face the adjacent column of molecules, unlike in 5.
These results suggest that the self-assembly of alkyl-decorated thiophene-containing species is sensitive to chain attachment chemistry and position. Detailed images and computational modeling will aid in interpretation of the affect these molecular changes have on the interactions between molecules and with the substrate. This work will offer a detailed understanding of the effects of molecular structure on packing and adsorption strength.
Two undergraduate students have carried out this work, Jolie Blake and Felicia Lucci. Both students grew intellectually as a result of performing this research, and have begun graduate work with interest in continuing in the field of surface chemistry.