www.acsprf.org
Reports: UR1049390-UR10: Enhancing Conversion Efficiency of Dye-Sensitized Solar Cells by Synthesis of Highly Ordered Titania Structures and Judicious Selection of Redox Couples
Feimeng Zhou, PhD , California State University (Los Angeles)
49390-UR10: Enhancing Conversion Efficiency of Dye-Sensitized Solar Cells by Synthesis of Highly Ordered Titania Structures and Judicious Selection of Redox Couples
Feimeng Zhou, California State University, Los Angeles
Summary:
We have (1) synthesized TiO2 nanowire array as the dye host, explored the blocking effectiveness of adsorbed silane and/or in situ polymerized phenol on the photogenerated charge recombination, and (2) optimized synthesis conditions to fabricate nanowire array with a large specific surface area and a high dye load.
Varying the precursor concentration, temperature and anisotropic adsorption agents, TiO2 nanowire array has been grown on conductive glass substrates. The array provides an excellent substrate for dye loading and surface modification so as to block the undesired side reactions and to optimize the energitics in dye sensitized solar cells.
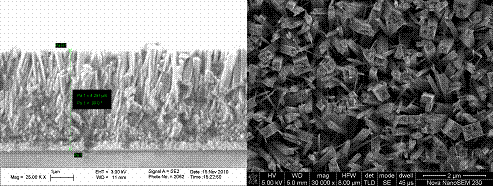
Fig. 1 SEM images of the TiO2 nanowire arrays: Cross-sectional (left) and top (right) views.
Fig. 1 shows a representative image of the TiO2 nanoarrays we synthesized. The width of the wire is about 100 nm and the length (thickness) about 4 µm. For efficient light harvesting, nanoarrays with a larger surface area, or thinner and longer wires are desirable. As such, one of the experiments we will conduct is to continue to optimize the synthetic conditions for obtaining optimal TiO2 nanoarrays. We have tried using water immiscible organic solvent, which helps dissolve the precursor and enhance the anisotropic growth due to the anisotropic adsorption of the organic solvent. It appears that thinner and longer wire arrays can be synthesized. We are optimizing the conditions and characterizing the array films synthesized in such way. This new procedure should allow us to grow TiO2 arrays for making high efficiency dye sensitized solar cells.
Another front of our research is to develop methods for effective insulation of the uncovered bare sites at the conductive substrate and the bare sites on TiO2 surface so that side recombination reactions of the injected electrons with the oxidized form of the redox species in solution can be prevented. To test the effectiveness of the surface modification, we have used the ferrocene/ferrocenium redox couple.
Two approaches have been developed for the surface inhibition. The first is to electrochemically deposit a layer of poly(phenylene oxide) and the second is to grow a monolayer of alkylsilane.
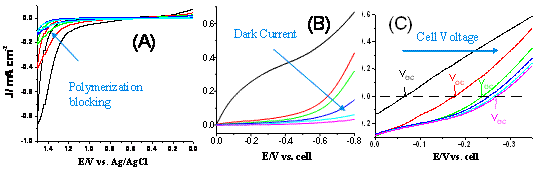
Fig. 2. I-V curves of the dye loaded TiO2 nanowire array electrodes in the phenol and ethenyl phenol solution (A), and in ferrocene/ferrocenium solution in the dark (B) and under illumination (C).
Fig. 2 (A) shows the I-V curves of the nanowire array electrode that has been blocked through electrochemical polymerization of poly(phenylene oxide). Clearly, with more electrochemical potential scans the phenol oxidation peak decreases, indicative of the formation of an insulation layer. This is also confirmed by the decreased dark current during the potential scan in a ferrocene/ferrocenium solution (Fig. 2 (B)). The effectiveness of the procedure has been demonstrated by the enhanced performance of the solar cell after the treatment. Shown in Fig. 2 (C) are the I-V curves of the solar cell before treatment and after varying numbers of treatments. Clearly the short circuit current and the open circuit voltage are both increased.
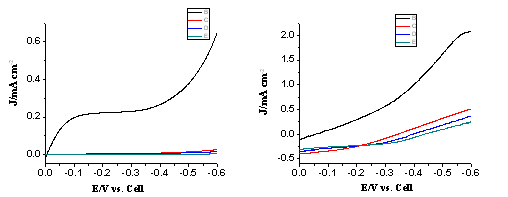
Fig. 3 The I-V curves of the electrode before silanization and after a few times of silanization treatments in the dark (left) and under illumination (right).
Fig. 3 shows the I-V curves of the electrode before and after silanization treatments. Clearly, the silanization step effectively insulates the bare sites of the electrode, as evidenced by the drastic decrease in the dark current of the electrode. The silanization also effectively blocks the recombination reaction between the injected electrons and ferrocenium in solution, as demonstrated by the increased short circuit current and open circuit voltages.