www.acsprf.org
Reports: UR349503-UR3: In Situ Thermodynamic Characterization of Supramolecular Assembly Processes that Lead to Discrete Nanosize Structures
Douglas A. Vander Griend , Calvin College
In our quest to study chemical systems with high degrees of organizational, dynamic, and compositional complexity, we have several new major scientific results to report this year. For details of previous results, please see the annual report submitted in September of 2010.
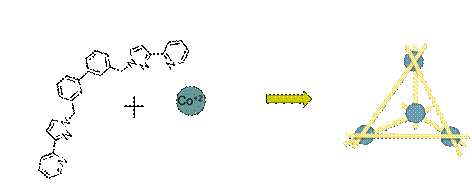
After establishing the usefulness of spectrophotometric titrations for investigating complicated solution dynamics, we took on our biggest challenge yet: monitoring the thermodynamic assembly of ten molecular pieces into a supramolecular tetrahedron (Figure 1). The original chemistry was designed by Dr. Michael D. Ward of Sheffield University, but neither he nor anyone else had studied the solution-phase assembly of this ten-piece nanocontainer.
Figure 1. Six Ligands (two bidentate pyrazolyl-pyridine termini separated by a 3,3'-biphenyl spacer) and 4 cobalt(II) cations form a tetrahedral nanocage in acetonitrile solution.
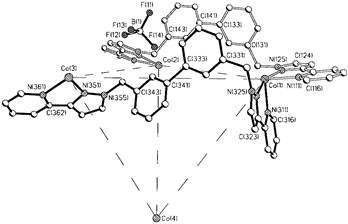
Figure 2. The supramolecular nanocage in the solid state has been previously characterized by X-ray diffraction.
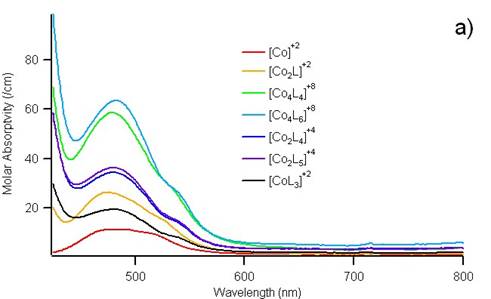
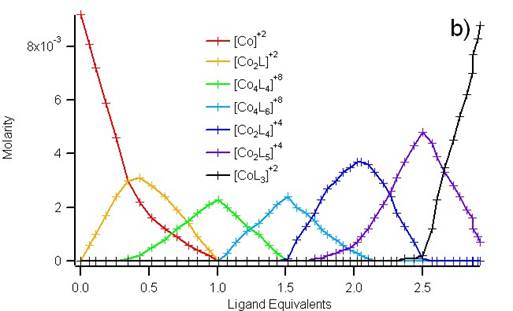
By titrating ligand into an acetonitrile solution of Co(BF4)2, the color was observed to shift only subtlety. However, spectroscopic measurements of the absorbance from 400 to 800 nm could be modeled to reveal the presence of 6 different supramolecular structures. Figure 3 shows the how the data deconvoluted to molar absorptivity spectra and concentration profiles for seven distinct species, including [Co(MeCN)6]2+.
Figure 3. The spectral data from approximately 50 different solutions of varying ratios of ligand and cobalt cation could be deconvoluted into molar absorptivity curves (a) and concentration profiles (b) for seven different absorbing species.
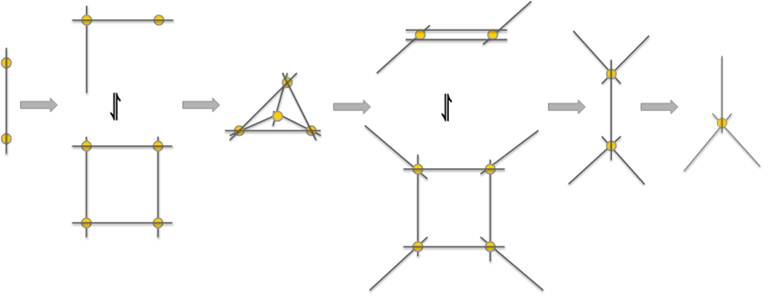
These data represent 'snapshots' of the assembly of the tetrahedral nanocontainer in which several distinct partial structures could be identified. Figure 4 depicts the expected structures of these different supramolecular complexes. These complexes are primarily identified by their stoichiometry and so in some cases there is some ambiguity. For example, when structures form with twice as many ligands as metal cations, the total structure could be a 4:2 combination or an 8:4 one. In reality there could be even more than two possibilities for such ratios and in fact multiple structures with the same empirical formula could well be in equilibrium with each other.
Figure 4. Upon titrating cobalt(II) with ligand in acetonitrile solution, Co2+ cations (yellow spheres) coordinate with the ditopic ligand (grey lines) to form an array of different supramolecular structures. In some case, the empirical formula allows for several reasonable structures which are represented as being in equilibrium with each other.
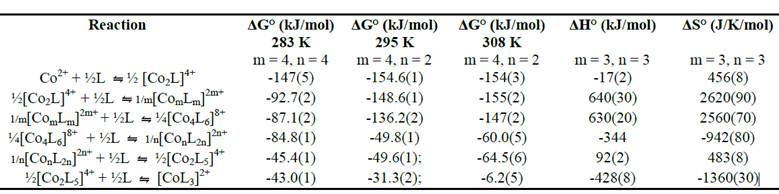
The modeling of the data also yields quantifiable thermodynamic parameters for the chemical reactions involved. Table 1 lists the different chemical reactions for the stepwise assembly and disassembly of the targeted nanocage along with the free energy (DG°) of the reaction. By running the titration repeatedly at various temperatures, enthalpy (DH°) and entropy (DS°) data could also be ascertained. Of major significance is the fact that the assembly process is entropy driven from both directions. This means that it is the freeing of the acetonitrile solvent molecules that help promote the assembly despite the aggregation of positive charge, which results from brining the cobalt dications into such close proximity to each other.
Table 1. Thermodynamic parameters determined via the modeling of spectroscopic data for the stepwise assembly of a cobalt(II) based supramolecular nanocontainer.
Despite the clear success of this study in elucidating the assembly process of such 3D supramolecular structures, it has also become clear that whereas complexes with different relative compositions can be readily resolved, those with the same empirical formula cannot. As expected according to Le Chatelier's principle, there is a dependence on total concentration, i.e. dimers are favored over monomers at higher overall concentrations, this dependence is weak and studying systems over a wide enough concentration range presents additional pragmatic issues. To tackle these issues we have begun to use mass spectrometry, which is the perfect complement to our present expertise in that it characterizes the mass and charge of discrete molecules/ions. In the coming years, we hope to demonstrate the use of these techniques together to unravel even more complicated solution-phase systems.
The impact of the research into equilibrium self-assembly over the last 12 months has been substantial. Three new undergraduate students joined my lab this summer and all testified that the experience definitely excited them for more research. They continue to conduct research now that the school year has begun and I expect that all three of them will return to my lab next summer.
As for my career, I continue to work with collaborator Dario Pasini of the University of Pavia, Italy; and Michael Ward of Sheffield University, England; as well as interact to various degrees with several other researchers who have asked for help in characterizing their particular ensemble of compounds. I continue to champion the power of these techniques as well as the wide ranging applications as I give seminars around the country.
Thank you for supporting this research and making the impact possible.