www.acsprf.org
Reports: DNI549596-DNI5: Structure and Dynamics of Aqueous and Aqueous-Hydrocarbon Fluids between Charged Surfaces
Yongsheng Leng, PhD , George Washington University
Clay swelling at different sedimentary basin conditions was studied in previous works1-3 . The present project focuses on the clay swelling mechanism in aqueous-hydrocarbon mixture and the adsorption structures of water, ion, and hydrocarbon species near charged clay surfaces. Specifically, we are interested in a fundamental problem: How does the clay swell in an aqueous environment from a dehydrated state.
Molecular dynamics (MD) simulations and the grand canonical Monte Carlo (GCMC) simulations were performed to study the swelling behavior of Na-montmorillonite. The model of clay used in this work is the sodium-saturated Wyoming-type montmorillonite Na0.75[Si7.75Al0.25](Al3.5Mg0.5)O20(OH)4. We use NPT molecular ensemble under the condition of 300K temperature and 1 atm pressure. The potential model used for the water molecule is the SPC flexible model4. The CLAYFF5 force field for clay minerals has been used in this work. The swelling curve obtained from the MD simulations is in close agreement with experimental results6, as well as with early MD simulation result<5. For the GCMC simulation, we tested different water chemical potential for different given clay spacing. The results show that when the chemical potential for water was set to be -20.786kJ/mol, the swelling curve was in agreement with the experimental results up to two hydration layers. When the chemical potential of water was increased to ¨C16.629kJ/mol to close to the bulk value, water content could continue increase at three layers and beyond. These results are shown in Fig. 1.
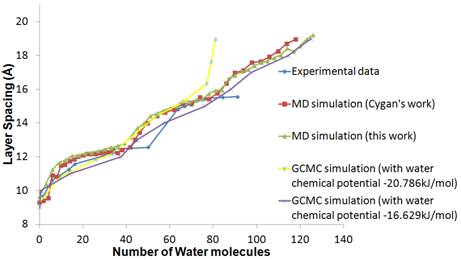 |
Fig. 1. Swelling curve of a Na-montmorillonite.
GCMC was further used to study the clay swelling in water-methane mixture with different chemical potentials of water and methane (µH2O and µCH4) to test some key studies in the previous work7-9>. The potential model used for the water molecules is the rigid SPC model and methane is represented by the OPLS-UA model. The chemical potential for pure water at T=300K is found to be about -45.73 kJ/mol. This value is approximately equal to the value for the TIP4P water in montmorillonite clay (-44.5 kJ/mol) . Different chemical potentials for methane have been tested, corresponding to different sedimentary condition. Our primary findings include (see Table I): (1) A threshold chemical potential for methane (about -17 kJ/mol) exists. Below this value, methane could not enter the interlayer of the clay; (2) Higher chemical potential for methane corresponds to higher pressure of the mixture in the interlayer, in which there is a higher possibility for methane to be present in the interlayer; (3) The relationship between the chemical potential of methane and its number fraction in water-methane mixture is non-linear.
Table 1. The µCH4µH2OVT ensemble simulation results for CH4 and H2O mixtures. The chemical potential for water at T=300K is set to be -45.73 kJ/mol. The clay spacing is 19 Å.
Chemical potential for methane (kJ/mol)
| -24.94
| -16.63
| -8.31
| +8.31
| +24.94
|
Number of methane molecules
| 0 | 5 | 30 | 36 | 49 |
Number of water molecules
| 123 | 110 | 68 | 65 | 46 |
Methane number fraction
| 0 | 0.0455 | 0.4412 | 0.5538 | 1.0652 |
To investigate the swelling phenomenon of clay from the dehydrated state and the exchange mechanism of different species between micropore and interlayer, a liquid-vapor canonical ensemble (LV-NVT) molecular simulation method has been proposed. The model will include the transport of different species between bulk micropore and interlayer spacing, dynamics of clay sheets allowing the clay expansion, and the lateral pressure control by forcing the liquid-vapor interfacial particles to meet the different sedimentary pressure conditions.
The project supports one PhD student to obtain substantial training in MD and GCMC molecular simulations. Further testing of the LV-NVT hybrid MC and MD simulation method against the GCMC simulation will enhance the PI's research development in aqueous-hydrocarbon systems.
(1) Sposito, G.; Skipper, N. T.; Sutton, R.; Park, S. H.; Soper, A. K.; Greathouse, J. A. Proceedings of the National Academy of Sciences of the United States of America 1999, 96, 3358-3364.
(2) Skipper, N. T.; Lock, P. A.; Titiloye, J. O.; Swenson, J.; Mirza, Z. A.; Howells, W. S.; Fernandez-Alonso, F. Chemical Geology 2006, 230, 182-196.
(3) Cygan, R. T.; Guggenheim, S.; van Groos, A. F. K. Journal of Physical Chemistry B 2004, 108, 15141-15149.
(4) Teleman, O.; Jonsson, B.; Engstrom, S. Molecular Physics 1987, 60, 193-203.
(5) Cygan, R. T.; Liang, J. J.; Kalinichev, A. G. Journal of Physical Chemistry B 2004, 108, 1255-1266.
(6) Fu, M. H.; Zhang, Z. Z.; Low, P. F. Clays and Clay Minerals 1990, 38, 485-492.
(7) Park, S. H.; Sposito, G. Journal of Physical Chemistry B 2003, 107, 2281-2290.
(8) Titiloye, J. O.; Skipper, N. T. Molecular Physics 2001, 99, 899-906.
(9) Titiloye, J. O.; Skipper, N. T. Journal of Colloid and Interface Science 2005, 282, 422-427.
(10) Tambach, T. J.; Bolhuis, P. G.; Hensen, E. J. M.; Smit, B. Langmuir 2006, 22, 1223-1234.
