www.acsprf.org
Reports: DNI649572-DNI6: Vibrational and Electronic Spectroscopy of Cationic Diamondoids
Nicolas C. Polfer, PhD , University of Florida
Initial Custom-Built Ion Deposition Instrument
The initial design of the custom-built ion deposition instrument, which allows a relative âsoft'-landing of mass-selected ions, is shown in Figure 1. The ion source includes the high electron emission current EI source, followed by an ion bender (Colutron Research Co.), a quadrupole mass filter, and an Einzel lens to guide the ions onto the deposition window. This instrument was capable of mass-selectively transmitting 3-4 nA of ion current to the Einzel lens after the quadrupole mass filter, when ions were biased at merely +8 VDC. This means that the ions would impact the matrix surface at a low kinetic energy (i.e., 8 eV).

Figure 1. Left: Schematic representation of initial version of custom-built ion deposition instrument, involving EI ion source, ion bender, quadrupole mass filter, Einzel lens and cryostat deposition window. Right: Photograph of instrument.
Instrument Improvements over Past Year
In order to increase ion transmission to the cryostat window, a number of instrumental modifications have been carried out over the past year, to result in the set-up shown in Figure 2:
1. A second ionization source for anions (- ions) has been installed. This is a chemical ionization (CI) source to produce counter-anions (e.g. SF6-).
2. The cation and anion beams are merged in a second bender and transferred through an octopole ion guide to the cryostat window held at 10 Kelvin.
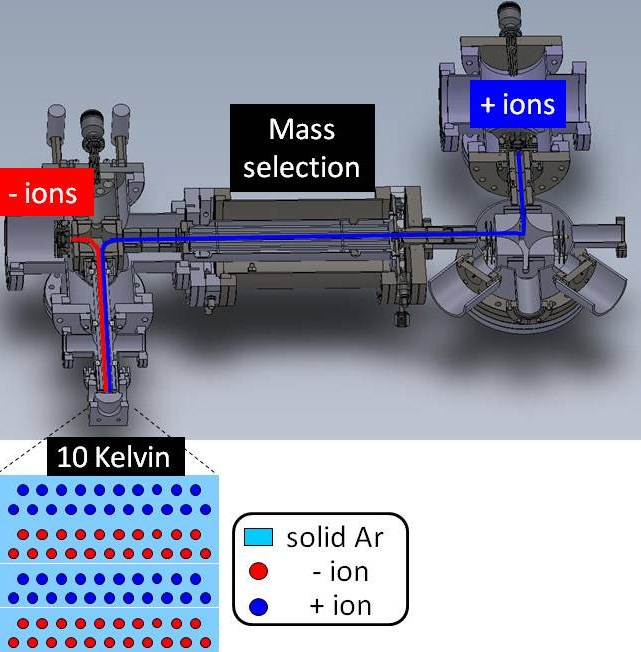
Figure 2. Schematic representation of extended version of custom-built ion deposition instrument, involving ion sources for cations (blue) and anions (red), where both ion beams are guided to the cryostat window to be imbedded in solid argon matrix at 10 Kelvin.
Preliminary Results
Simultaneous desposition of cations (e.g. CO2+ ) and anions (e.g. SF6- ) was carried at current intensities of 2-3 nA for 24 hours.
Despite the considerable deposition times, no infrared (IR) absorption bands were detected that could be assigned to ions. Conversely, abundant IR bands assigned to neutral SF6 and CO2 could be discerned. This suggests that the cations and anions engage in electron transfer neutralization reactions. In order to prevent cation-anion recombination reactions, the cations and anions will be deposited separately in layers, as shown in the inset in Figure 2. It is expected that this approach will result in high ion densities in the matrix, while minimizing recombination reactions. We are currently in the process of implementing pulsed DC power supplies to enable this approach.
Scientific Meetings
The graduate student on this project, Nathan Roehr, has presented updates on this custom-built instrument at the annual meeting of the American Society for Mass Spectrometry [1].
References
[1] Roehr, N.; Szczepanski, J.; Polfer, N.C.; FTIR experiments on mass-selected cations by counter-ion introduction into an inert argon matrix. 59th American Society for Mass Spectrometry Meeting (Denver, CO, June 5-9 2011).
