www.acsprf.org
Reports: UNI150058-UNI1: Elaboration of Petroleum-Derived Aromatics for the Generation of Conjugated Discotic and Cruciform Structures: Controlling Electronic Properties via Transition Metal Coordination
Nathan P. Bowling, PhD , University of Wisconsin (Stevens Point)
Conjugated molecules are known to have interesting electronic properties that can potentially be useful in the development of functional organic materials. To function as a conjugated system, a molecule must fit two criteria. First, a molecule must have π-systems on adjacent atoms that are capable of interacting. Second, these orbitals must be aligned properly to allow for electron transport/delocalization between orbitals. A key motivating factor in our research program is to develop molecules that fit the first criteria, but only achieve the second with some external stimulus. In our current research efforts, this forced alignment of π-systems is achieved via transition metal coordination.
 Over
the course of the last two years, six students have worked on the synthesis of
structures 1 and 2. In this time, they have been able to develop
relatively high yielding synthetic protocols for these two compounds. Because 1
and 2 are acyclic, there should be free rotation of many of the single
bonds within these molecules. This free rotation will inhibit the effective
conjugation of these unsaturated systems. Bidentate coordination of these
ligands to transition metals will result in a cyclic metallorganic structure
which will no longer suffer from free rotation. Therefore, electronic
delocalization of these complexes should be maximized relative to the acyclic
precursors.
Over
the course of the last two years, six students have worked on the synthesis of
structures 1 and 2. In this time, they have been able to develop
relatively high yielding synthetic protocols for these two compounds. Because 1
and 2 are acyclic, there should be free rotation of many of the single
bonds within these molecules. This free rotation will inhibit the effective
conjugation of these unsaturated systems. Bidentate coordination of these
ligands to transition metals will result in a cyclic metallorganic structure
which will no longer suffer from free rotation. Therefore, electronic
delocalization of these complexes should be maximized relative to the acyclic
precursors.
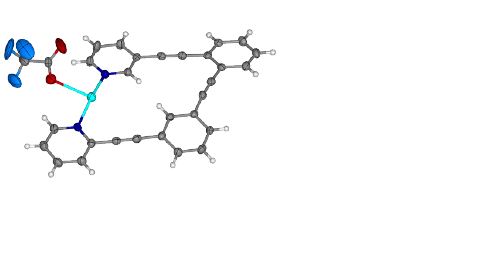
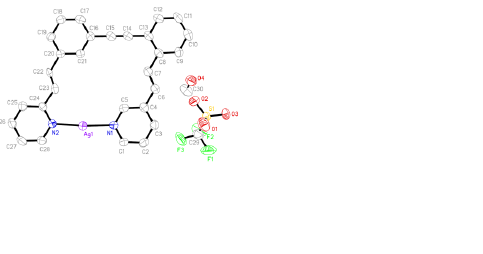 Metal
coordination is clearly indicated by changes in the 1H NMR chemical
shifts of 1 and 2 upon introduction of Pd(II) or Ag(I). The
difficulty with NMR characterization, however, is that it does not distinguish
between the predicted cyclic metallorganic structures and open chain
coordination polymers. In order to address this issue, we have focused our
efforts on obtaining X-ray crystal structures of the proposed complexes.
Initially, my students were growing crystals and sending samples to off-site
labs for analysis. This method was impractical as transport and subsequent analysis
of the crystals (that are air and light sensitive) in a timely fashion was
difficult. Fortunately, we have been able to establish a collaboration with
Eric Bosch (Missouri State University), who is able to grow and analyze
crystals of these complexes in his research facility. This has allowed us to
send ligands 1 and 2 (which are very stable) via mail without
worries of decomposition. The success of this collaboration is immediately
evident from the crystal structures that have been generated in a very short
period of time.
Metal
coordination is clearly indicated by changes in the 1H NMR chemical
shifts of 1 and 2 upon introduction of Pd(II) or Ag(I). The
difficulty with NMR characterization, however, is that it does not distinguish
between the predicted cyclic metallorganic structures and open chain
coordination polymers. In order to address this issue, we have focused our
efforts on obtaining X-ray crystal structures of the proposed complexes.
Initially, my students were growing crystals and sending samples to off-site
labs for analysis. This method was impractical as transport and subsequent analysis
of the crystals (that are air and light sensitive) in a timely fashion was
difficult. Fortunately, we have been able to establish a collaboration with
Eric Bosch (Missouri State University), who is able to grow and analyze
crystals of these complexes in his research facility. This has allowed us to
send ligands 1 and 2 (which are very stable) via mail without
worries of decomposition. The success of this collaboration is immediately
evident from the crystal structures that have been generated in a very short
period of time.
When mixed with AgOTf or Pd(PhCN)Cl2, our ligands form bidentate complexes. As predicted, the three crystal structures above indicate a structure constrained to planarity via transition metal complexation. The only crystal structure that has not been obtained so far is that of 1 with Pd(II). We anticipate having this structure in the very near future. The manuscript that will communicate these results to the chemistry community is in preparation. There will be six student authors from UWSP along with authors from Missouri State University. Of the student authors that have worked on this project, three have graduated, and three are current students. One graduate is in pharmacy school at the University of Wisconsin-Madison. Another (current status unknown) was seeking employment in chemical industry upon graduation. The third graduate is now participating in AmeriCorps, and anticipates applying to graduate programs in biochemistry, or a related area, in the future. Two current students are pursuing careers in health sciences. The third current student is planning on applying to medicinal chemistry graduate programs this winter.
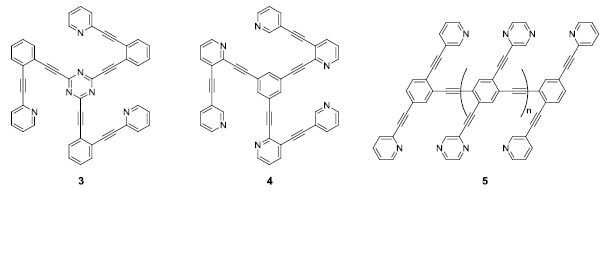
Ultimately, we envision incorporating these types of simple conjugated units (1 and 2) into larger conjugated structures (e.g. 3, 4 and 5). Five students (three of them mentioned above) have been attempting to generate these molecules. The two students that were not discussed above worked on this project between their freshman and sophomore years. One is a chemistry major, while the other is a biochemistry major. They will likely continue working on these projects until the targets are successfully acquired or the students graduate.
The high symmetry and extended conjugation in these structures makes them difficult to isolate and purify. Generation of 3 and 4, for example, requires three concurrent Sonogashira coupling reactions. Incomplete conversion can lead to mono-coupled and di-coupled by-products, which are nearly impossible to separate chromatographically from the desired tri-coupled product. Extended conjugation within both the target molecules and their precursors makes the synthesis and isolation of these challenging. We have avoided some of these difficulties by incorporation of dodecyloxy or tert-butyl solubilizing groups. Synthetic efforts toward these targets will continue through this academic year and through the following summer.