www.acsprf.org
Reports: UNI649343-UNI6: Room Temperature Chirped-Pulse Fourier Transform Microwave Spectroscopy for the Study of Radical Reaction Dynamics
Steven T. Shipman, PhD , New College of Florida
The goal of this project is to use chirped-pulse Fourier transform microwave (CP-FTMW) spectroscopy from 8.7 18.5 GHz as a probe of gas phase chemical reactions occurring at low pressures (1 100 mTorr) and at room temperature. The original proposal discussed reactions between small unsaturated hydrocarbons (such as propene) and hydroxyl radicals, created from hydrogen peroxide by pulses of UV light.
All of these measurements utilize the CP-FTMW spectrometer at New College of Florida. Funding from ACS-PRF has provided a UV light source, 0.3-, 0.6-, and 2-meter long waveguide sample cells, and optics and vacuum components used to transmit light and molecules into and out of the sample cells. To date, this award has supported four undergraduates for 10 weeks of summer research each: Benjamin Kriegel (2009), Sophie Lang (2010), and Morgan McCabe and Maria Phillips (2011). Ben started chemistry graduate school this fall at UC-Berkeley. Sophie and Maria intend to graduate with a B.A. in Chemistry in Spring 2013, and Morgan intends to graduate with a B.A. in Chemistry in Spring 2014.
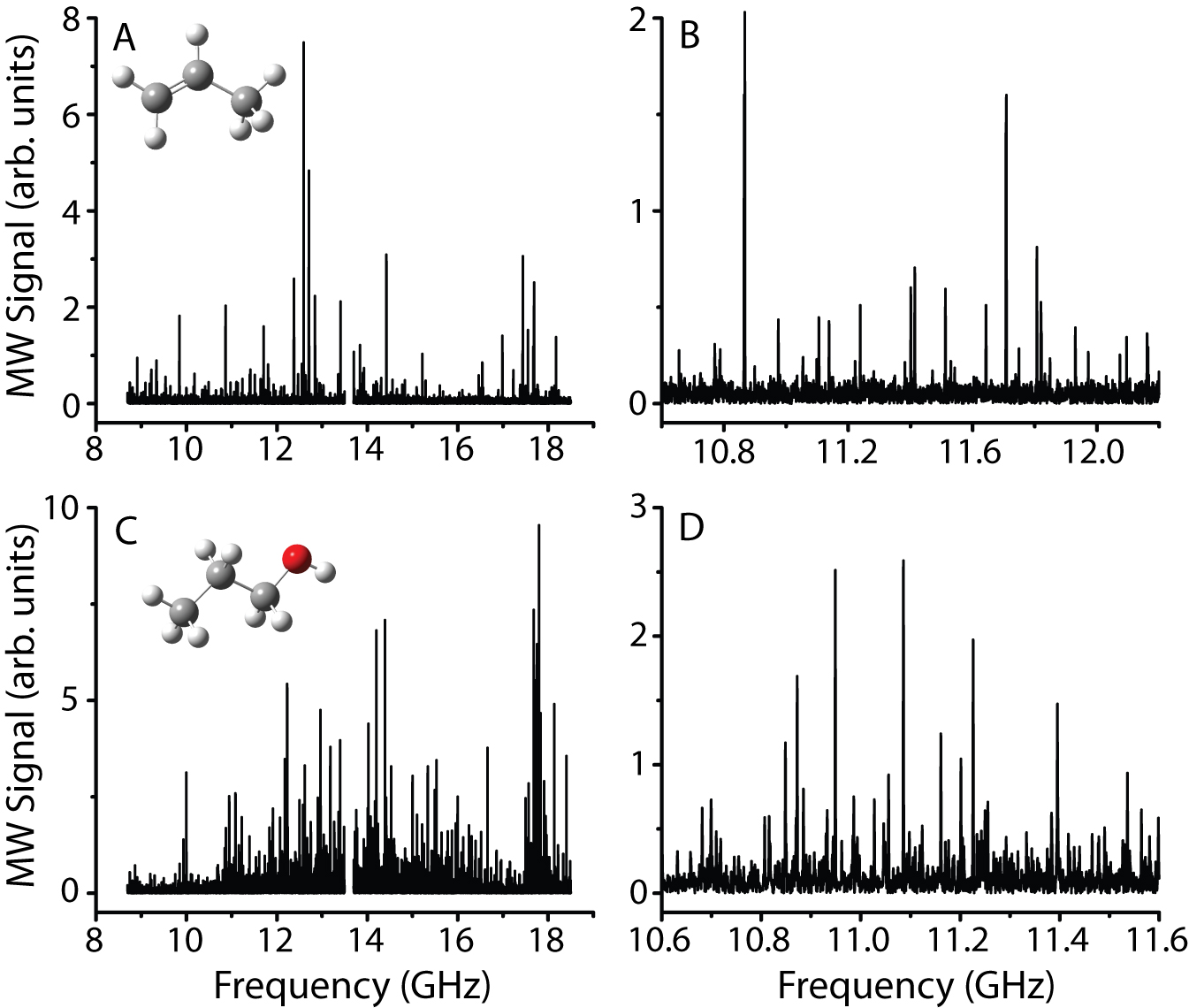
Work began on this project during the summer of 2009, during which we performed initial characterizations of the unsaturated hydrocarbon reactants and closed-shell forms of some of the potential products. Ben Kriegel collected and analyzed the room-temperature rotational spectra of seven molecules propene, 1-butene, isoprene, 1-propanol, 2-propanol, 1-butanol, and 2-butanol. As the New College spectrometer had only been commissioned a few months prior, these measurements also served to test the instrument sensitivity. Figure 1 shows a few of the spectra that Ben collected. As a side benefit, these spectra were of interest to spectroscopists who are searching for organic molecules in the interstellar medium. As the New College spectrometer operates in a unique temperature and frequency range, our observations nicely supplemented existing data from millimeter-wave instruments, and these results have been published in Physical Chemistry Chemical Physics.
During the summer of 2010, Sophie Lang worked in the lab to implement schemes for microwave-microwave double resonance and to incorporate the UV source into the experimental setup. Her double resonance work, which effectively tags spectral features that share a quantum state with a pumped feature, was successful. Reaction initiation with the flash lamp was less successful. Her initial plan was to monitor depletion of the propene signal from a propene / hydrogen peroxide gas mixture as a function of UV exposure. Early attempts at these measurements were unsuccessful, and so that fall we devoted time to determining why. We built new microwave circuits to enhance the signal-to-noise ratio of the propene signal and obtained new optics to better focus and collimate the UV light from the flash lamp. We eventually determined that the hydrogen peroxide was reacting with the waveguide walls in the absence of UV, consuming all of the hydrogen peroxide and preventing reactions from taking place with the alkene. Based on this, we devoted the spring and summer of 2011 to finding new candidates to replace the hydrogen peroxide. We needed candidate molecules that had reasonable dipole moments, were stable in the dark after repeated collisions with the walls of the sample cell, and would easily dissociate under UV irradiation. Morgan McCabe and Maria Phillips tackled this problem by considering all possible two- and
Figure 1. Panels A and B (expanded view of A) show the room-temperature microwave spectrum of propene, and panels C and D (expanded view of C) show the room-temperature microwave spectrum of 1-propanol. Each spectrum represents roughly 16 hours of data collection.
three-carbon-containing molecules with at least one CCl, CBr, or CI bond. They performed ab initio calculations on each candidate to determine dipoles, rotational constants, and vibrational partition functions at room temperature. They used these values along with price and availability to settle on four candidates: allyl chloride, allyl bromide, iodoethane, and 1-chloropropane.
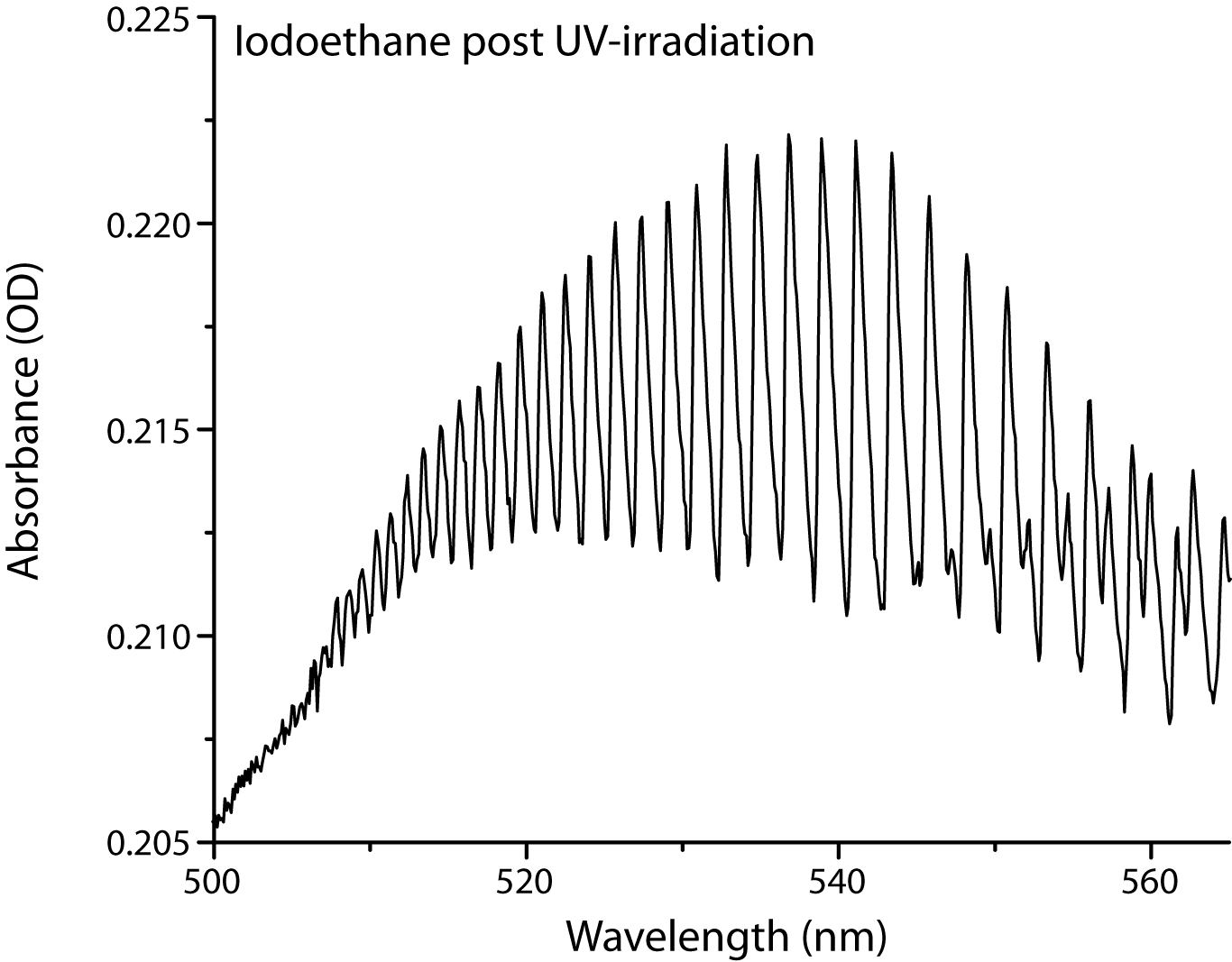
They obtained spectra for each molecule (an analysis of the allyl chloride spectrum (Figure 2) was presented at the 66th International Symposium on Molecular Spectroscopy) and verified that the molecules would react under UV light by filling a small gas cell with a few Torr of the compound and taking UV and FTIR spectra before and after irradiation. After irradiating iodoethane for 30 minutes, its UV-Vis spectrum (Figure 3) shows clear evidence of the presence of I2, indicating that the CI bond is breaking after exposure to UV light. Unfortunately, equipment failures of microwave components and a pulsed delay generator during the summer prevented us from initiating reactions in the microwave sample cell, but those are the first experiments we plan to perform when the repaired equipment is returned.
Figure 2. The rotational spectrum of allyl chloride, a suitable precursor for forming UV-induced radicals. Undergraduate students assigned the spectrum and the results were presented at the 66th International Symposium on Molecular Spectroscopy.
Figure 3. UV-Vis spectrum of iodoethane, a colorless gas, after 30 minutes of UV irradiation, showing the clear formation of I2. The CI bond in iodoethane is breaking as a result of UV exposure, indicating that it should be a good candidate for the proposed MW measurements.
In addition to the work described above, materials and equipment provided by this award have been used by New College students as part of their senior thesis projects that are required for graduation. One student, Ian Finneran (currently a graduate student at CalTech) obtained and analyzed the spectrum of anisole, 2-methylfuran, and o-fluorotoluene with the microwave spectrometer. The results from the anisole study have been submitted to the Journal of Molecular Spectroscopy (papers from the other molecules are in preparation), and data from both his work and Sophie Lang's work carried out under this award were used as part of a successful proposal to the NSF's Chemical Measurement and Imaging program (award #1111101).