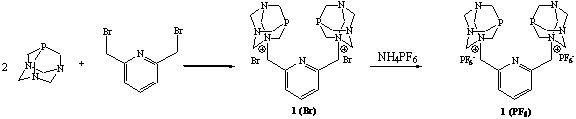www.acsprf.org
Reports: B346716-B3: Synthesis, Coordination Chemistry, and Catalytic Studies of a Library of Water-Soluble Polydentate Phosphines Derived from 1,3,5-Triaza-7-phosphaadamantane (PTA)
Donald A. Krogstad , Concordia College
Over the past three decades, multinuclear metal complexes have shown great promise as effective catalysts. These compounds are traditionally prepared by employing bridging ligands, particularly P,P systems. Unfortunately, many of the bis-phosphines are only soluble in environmentally hazardous solvents. Therefore, our lab recently started to prepare a library of P,P ligands that are water-soluble. These were the first compounds to employ two PTA (1,3,5-triaza-7-phosphaadamantane) moieties. PTA was an advantageous synthon as it is air-stable, resistant to oxidation, and highly water-soluble (235 mg/mL). The previously prepared ligands all contained an aromatic linkage that was believed to minimize the water-solubility of their metal complexes.
To add to this collection of P,P ligands, and to increase the water-solubility, the pyridyl derivative, 1,1'-[1,3-pyridylenebis(methylene)]bis-3,5-diaza-1-azonia-7-phosphatricyclo[3.3.1.1]decane dibromide (1), was prepared in high yields by reacting two equivalents of PTA with 1,3-bis(bromomethyl)pyridine in dry acetone (Scheme 1). The PF6- analogue of 1 was prepared by a metathesis reaction involving NH4PF6. This same approach was used to synthesize the PF6- salts of the original P,P ligands that contained phenyl, tolyl, and anisolyl backbones. Unfortunately, the counter-ion substitution had a negative impact on the water-solubility of the ligands. For example, the bromide salt of 1 was found to have a solubility of 1600 mg/mL while the solubility of the PF6- analogue was only 110 mg/mL. Even though the solubility was decreased, it was important to possess these systems as they allowed us in the coordination studies to utilize common chloride metal salts and not produce mixed halide complexes.
Scheme 1. Synthesis of bis-phosphines 1(Br) and 1(PF6).
In an effort to expand the coordination chemistry of the previously prepared tolyl-P,P ligand, 1,1'-[1,3-tolylenebis(methylene)]bis-3,5-diaza-1-azonia-7-phosphatricyclo[3.3.1.1]decane dihexafluorophosphate (2), as well as to explore the reactivity of 1 (PF6), the bis-phosphines were combined with AuCl(THT) (THT = tetrahydrothiophene, Scheme 2A), K[PtCl3(C2H4)] (Scheme 2B), cis-[MCl2(COD)] (M = Pt, Pd; COD = 1,5-cyclooctadiene, Scheme 2C), and cis-[MCl2(DMSO)2] M = Pt, Pd; DMSO = dimethylsulfoxide, Scheme 2D). Multinuclear NMR and ESI-MS indicated that 1 and 2 acted as bridging ligands to form the binuclear complexes [AuCl(m-P,P)AuCl](PF6)2 (3, P,P = 1 and 2), cis,cis-[PtCl2(m-P,P)2PtCl2](PF6)4 (5, P,P = 1 and 2), and cis-[PtCl(DMSO)(m-P,P)2PtCl(DMSO)](PF6)4 (6, P,P = 1 and 2) respectively. It is interesting to note that eventhough alkene dissociation from Zeise's salt and [MCl2(COD)] readily opens one and two coordination sites respectively, both synthons, regardless of metal: ligand stoichiometry, formed a bis P,P complex, 5. It was originally envisioned that a binuclear complex containing only one P,P ligand would be formed from K[PtCl3(C2H4)] as was observed with AuCl(THT). The 31P NMR spectra of the Pt compounds contained a series of doublets with 195Pt satellites of approximately 3500 Hz. The consistency of this 1JPt-P coupling constant indicated that the identity of the aromatic backbone did not greatly impact the basicity of the P,P ligands. This theory was further confirmed with the IR spectra of the DMSO complexes, 6. The Pt compounds exhibited identical SO stretching frequencies of 1122 cm-1 while the Pd complexes produced a band at 1072 cm-1. Again, this constant spectroscopic signal indicated that variation in the aromatic backbone did not change the nucleophilicity of the P,P ligand.
The gold compounds, 3, were further derivatized by allowing them to react with 1-thio-beta-D-glucose tetraacetate in the presence of base (Scheme 2E). The resulting binuclear, Au complexes, 4, were important because they were analogs of the antirheumatic drug Auranofin, and also because they possessed a tightly bound thiolate. This change in ligand basicity proved to be important in the catalytic activity of the complexes, and hence in the understanding of water-soluble catalyst design.
Scheme 2. Synthesis of binuclear metal complexes bridged by bis-PTA phosphines.
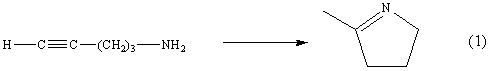
The catalytic abilities of the binuclear complexes 3-6 were studied by employing them in the intramolecular hydroamination /cyclization of 4-pentyn-1-amine into 2-methyl-pyrroline in water (Reaction 1).
Kinetic data were measured via 1H NMR under homogeneous conditions at 50oC with 0.5 mol catalyst loading (Table 1) and showed the following trends in rate: i) Au and Pd complexes produced faster rates than Pt complexes. (ii) Substituion of the chloride ligand for a less labile ligand such as thiolate or DMSO retarded the catalysis. (iii) The identity of the aromatic backbone had little effect on the catalytic rate. The most important data that were obtained related to the gold catalysis. This was the first study to illustrate that Au(I) complexes may be employed as effective aqueous phase intramolecular hydroamination catalysts. It also showed that binuclear Au catalysts may be superior to those that utilize only one metal center.
Table 1. Kinetic Data of the Catalyzed Hydroamination of 4-pentyn-1-amine in water at 50oC.
Complex
| Aromatic Backbone
| % Conversiona |
3
| pyridyl
| 93
|
3
| tolyl
| 90
|
4
| pyridyl
| 36
|
4
| tolyl
| 59
|
AuCl(PTA)
| none
| 88
|
[AuCl(pymePTA)]PF6b | pyridyl
| 52
|
5 (Pt)
| pyridyl
| 36
|
5 (Pt)
| tolyl
| 38
|
5 (Pd)
| pyridyl
| 85
|
5 (Pd)
| tolyl
| 78
|
6 (Pt)
| pyridyl
| 28
|
6 (Pt)
| tolyl
| 41
|
6 (Pd)
| pyridyl
| 93
|
6 (Pd)
| tolyl
| 90
|
a Determined by integration of the 1H NMR spectra at t=48 hours.
b The ligand [pymePTA]PF6 is the mono-PTA analog of 1.
Through this study, a new series of water-soluble binuclear Au, Pt, and Pd complexes have been prepared and examined as catalysts for the intramolecular hydroamination reaction. This work clearly showed that binuclear complexes have the potential to be effective catalysts for this important reaction, and also that Au may be a superior choice for the transformation. Most importantly, however, the research has been fruitful because it impacted the lives of the undergraduates involved. Three of the students involved will graduate in the spring of 2012, and all plan to attend graduate school to study either inorganic chemistry or material science. In fact, one student stated that this experience has changed his professional goals. He initially wanted to be a physician, but after his time in the lab, he wants to be a chemist. As he put it, “I can have a larger impact on people by developing pharmaceuticals than by simply prescribing them.”