www.acsprf.org
Reports: UR1050070-UR10: Controlling Morphology in Bulk Heterojunction Solar Cells via Chemical Design and Surface Patterning Methods
Lee Y. Park, PhD , Williams College
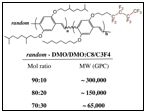 We have now made two types of partially
fluorinated PPVs. Type 1
fluoropolymers are the result of random copolymerizations between a GILCH type
monomer with hydrocarbon sidechains and an analogous monomer containing a
fluorinated sidechain. In preparing our Type 2 fluoropolymers, we used GILCH
type monomers with differing reactivities (bromomethyl for the monomer with
hydrocarbon sidechains vs chloromethyl for the monomer with fluorocarbon
sidechains), in the hopes that we might prepare polymers with a "blockier" type
of structure. We see a number of differences between the parent all hydrocarbon
polymers (MDMO-PPV) and our fluorinated derivatives. First, during the polymerizations, the polymers containing
the fluorinated sidechains are considerably darker in color. Second, the solubility of the polymers
decreases dramatically with the amount of fluorinated monomer present, with the
result that soluble high MW polymers (as determined via GPC) suitable for spin-coating
can only be obtained with relatively low mole percent of fluorinated monomer,
as shown in the table to the right.
Even for polymers with only 30 mole percent of the fluorinated monomer,
the polymers are highly insoluble.
We have now made two types of partially
fluorinated PPVs. Type 1
fluoropolymers are the result of random copolymerizations between a GILCH type
monomer with hydrocarbon sidechains and an analogous monomer containing a
fluorinated sidechain. In preparing our Type 2 fluoropolymers, we used GILCH
type monomers with differing reactivities (bromomethyl for the monomer with
hydrocarbon sidechains vs chloromethyl for the monomer with fluorocarbon
sidechains), in the hopes that we might prepare polymers with a "blockier" type
of structure. We see a number of differences between the parent all hydrocarbon
polymers (MDMO-PPV) and our fluorinated derivatives. First, during the polymerizations, the polymers containing
the fluorinated sidechains are considerably darker in color. Second, the solubility of the polymers
decreases dramatically with the amount of fluorinated monomer present, with the
result that soluble high MW polymers (as determined via GPC) suitable for spin-coating
can only be obtained with relatively low mole percent of fluorinated monomer,
as shown in the table to the right.
Even for polymers with only 30 mole percent of the fluorinated monomer,
the polymers are highly insoluble.
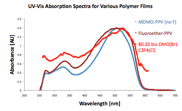
There are distinct shifts in the UV/Vis spectra for the different families of polymers. These can be observed both during the polymerizations as well as in films cast after purification. Shown here are UV/Vis spectra for films cast onto glass substrates. The curve in blue is for the parent MDMO-PPV; the darker red trace is for the Type 1 fluoropolymer, and the bright red trace is for the Type 2 fluoropolymer. Both types of fluorinated polymer clearly show a much broader absorption envelope, extending both to longer and shorter wavelengths relative to the parent polymer. Williams undergraduates who have worked on this project this year are: All work has been carried out by the following Williams College undergraduates: Cameron Rogers '12, Dan Gross '12, Grace Babula '12, Heather Burrell '11, Katrina Tulla '11, Erica Wu '13.