www.acsprf.org
Reports: DNI1050201-DNI10: Fundamental Studies of Lithiation/Delithiation Processes in V2O5 and MxV2O5 Nanostructures
Sarbajit Banerjee, PhD , State University of New York at Buffalo
1. Overview
The last decade of frenetic battery research has made clear the formidable challenges of safely achieving high power and energy densities in Li-ion batteries, which necessitate the optimal performance of disparate materials at the limits of their thermodynamic stability windows. Despite the high stakes, materials discovery approaches for developing cathode materials remain primarily empirical. A major impediment to the development of rational materials design and optimization approaches has been lack of fundamental mechanistic understanding of the transformations induced within materials upon lithiation (and subsequent delithiation). A major focus of our ACS PRF-funded research program has been to elucidate the influence of finite size, morphology, and stoichiometry on Li-ion insertion/extraction and diffusion processes in binary and ternary vanadium oxides, especially structures that crystallize in quasi-layered phases such V2O5 and MxV2O5.
Over the last year, we have mounted a systematic effort aimed at growing well-defined V2O5 and MxV2O5 nanostructures with a high degree of compositional, dimensional, and morphological control. We have further undertaken a comprehensive study of the geometric and electronic structure of these materials using both ensemble and individual nanowire spectroscopy and microscopy tools. Finally, we have performed a detailed mechanistic study of alterations to the geometric and electronic structure of Ag2VO2PO4 upon cathodic discharge, using X-ray absorption spectroscopy to develop an orbital-specific picture of the redox chemistry accompanying electrochemical lithiation.
2. Findings
2.1. Morphology Controlled Fabrication of V2O5 Nanostars: VO is the rare vanadium oxide to crystallize in a highly symmetric rock salt structure. In an article featured as the cover of CrystEngComm (Fig. 1), we have devised a novel hydrothermal synthetic approach that takes advantage of the intrinsic octahedral symmetry of rock-salt-structured VO to facilitate the growth of six-armed nanocrystallites of V2O5. A vapor transport process is used to first deposit a VO precursor onto a 100 nm Fe film. Selective etching of the deposited grains along high-surface-energy crystallographic facets yields V2O5 nanostructures that exhibit clear six-fold symmetry and retention of electronic structure. The lithiation/delithiation of these high-surface-area materials is currently being examined using Raman spectroscopy.
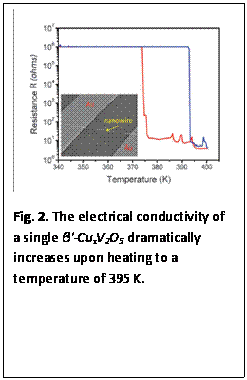
2.2. Single-Nanowire Measurements of β'-CuxV2O5 Nanowires: In keeping with our theme of combining ensemble characterization with individual nanowire electrical transport and electronic structure studies, we have examined the properties of β'-CuxV2O5 (x ~ 0.65) nanowires prepared by the hydrothermal reduction of CuV2O6. Studying single wires allows us to access the intrinsic structural and electronic properties of this 1D bronze material without obscuration from grain boundary connectivity or domain dynamics. Remarkably, we have observed a thermally induced transition to a metallic state characterized by very high conductivity in the 340—400 K range (Fig. 2) for single nanowires of these materials, likely arising from the melting of charge ordering (discrete V4+ and V5+ sites). The implications of a heating-induced enhancement in conductivity are significant for Li-ion batteries that need to maintain electroactivity at high ambient temperatures. Individual-nanowire scanning transmission X-ray microscopy measurements suggest that the magnitude of the phase transition depends sensitively on the precise stoichiometry of the nanowires.

|
|
2.3. Mechanistic Insights into the Cathodic Discharge of Ag2VO2PO4: Inspired primarily by the greater chemical stability of PO43- constituent building blocks, substantial recent research attention has focused on the use of phosphate derivatives of transition metals as cathode materials. Indeed, the MTM—O—P coupling of strongly covalent phosphate tetrahedra confers thermal and chemical stability to a variety of crystal structures. Ag2VO2PO4 has attracted particular interest as a candidate cathode material for use in the next generation of lithium-based batteries owing to its bimetallic nature, which faciliates multielectron transfer processes. A remarkable 15,000-fold enhancement in conductivity of this material is observed upon lithiation due to the extrusion of a percolative Ag nanoparticle network. However, the concomitant amorphization of Ag2VO2PO4 during electrochemical discharge precludes the use of standard diffraction tools and renders fundamental mechanistic studies of the lithiation process rather difficult.
X-ray absorption spectroscopy on the other hand poses no crystallinity requirements, and consequently in a comprehensive article published in the Journal of Physical Chemistry C, we have used a combination of V K-, V L-, Ag K-, and O K-edge X-ray absorption fine structure spectroscopy measurements to determine the local vanadium and silver oxidation states, local coordination geometry, and stoichiometry for Ag2VO2PO4 samples with varying extents of electrochemical lithiation. Hard-X-ray V K-edge X-ray absorption near edge structure (XANES) and Ag K-edge XANES and extended X-ray absorption fine structure (EXAFS) measurements suggest the concurrent and not sequential reduction of the Ag+ and V5+ redox-active sites of Ag2VO2PO4, although complete reduction of Ag+ to nanoparticulate Ag0 metal precedes the appearance of a significant concentration of V3+ species in the discharged phases. These measurements further enable a quantitative description of the stoichiometry and redox nature of samples at varying depths of discharge. Soft X-ray V L- and O K-edge NEXAFS spectroscopy in conjunction with density functional theory (DFT) calculations provide the first description of the electronic structure of Ag2VO2PO4 and evidence the stability of phosphate groups to advanced states of discharge and reduced covalency of V—O linkages. These specific structural characteristics make this system an attractive cathode material. The NEXAFS measurements further provide an exquisitely detailed picture of orbital occupancies as a function of the depth of discharge and directly evidence V—O rehybridization accompanying the electrochemical lithiation of Ag2VO2PO4. The sequence of V 3d states occupied upon electrochemical reduction from lower to higher energy follows: V 3dzy then 3dzx, and finally 3dxy. The methodologies developed here are applicable in principle to any cathode material without any crystallinity requirements to obtain a detailed picture of how geometric and electronic structure varies as a function of electrochemical lithiation and will be furher used to develop detailed mechanistic undertstanding of lithiation/delithiation processes in binary and ternary vanadium oxides.
