
ACS PRF | ACS
All e-Annual Reports

43265-GB6
Heterogeneous Chemistry of Gas-Phase Oxidants and Organic Aerosols
Significant interest in the oxidative ageing of atmospheric aerosols has driven project selection this past year to include heterogeneous chemical reactions of ozone with aerosols containing polycyclic aromatic hydrocarbons (PAHs), multi-component aerosols and spectral characterization of humic like substances found in atmospheric aerosols. Little is known about the oxidative ageing of particulate PAHs. In our studies, pyrene was selected as a model PAH. This compound is present in atmospheric aerosols and is relatively non-toxic compared other species in this class. Pyrene aerosols were generated using homogeneous nucleation; an argon stream is passed through a heated flask saturated with vapor and into a nucleation chamber. This process proved very challenging as the outlet of the heated flask was easily clogged by nucleating aerosols. Analysis of the reaction of pyrene aerosols with ozone demonstrated that a high concentration of ozone was necessary to see appreciable product formation. This may indicate that these reactions will not occur to a relevant extent in the atmosphere.
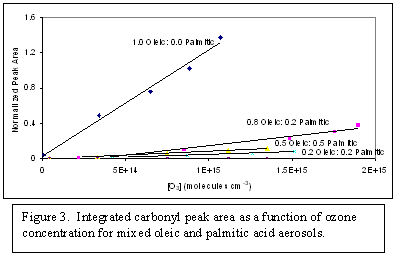
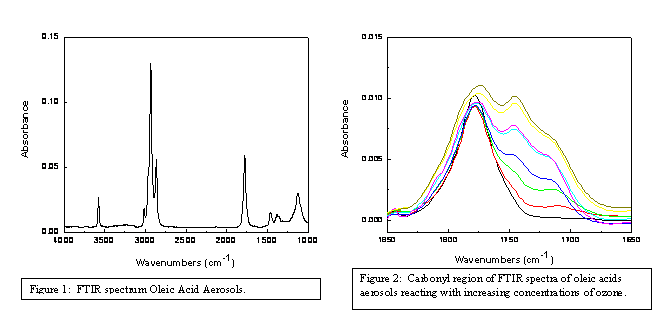 The second project, heterogeneous chemistry of multi-component aerosols, has provided some very exciting preliminary results. Oleic acid aerosols were generated using the pneumatic nebulizer and reacted with different concentrations of ozone at a constant residence time in the flow cell. A recent review of studies of this heterogeneous reaction system indicates that observed product species include the primary organic acid and aldehyde products with additional evidence of products from the secondary chemistry of the Criegee intermediates. One of the first challenges faced in studying the ozonolysis of oleic acid with the AFT-FTIR, developed as part of this proposal, is that oleic acid contains a carboxylic acid functional group prior to ozonolysis (Figure 1) and could the aldehydes and products from the secondary chemistry be distinguished from the initial carboxylic acid absorption feature? Absorption features attributed to aldehyde and ester functionalities are observed indicating the presence of primary and secondary products (Figure 2). Integration of the carbonyl region, normalized to the initial absorbance, demonstrates a linear increase in the extent of reaction as a function of ozone exposure. Other studies have also shown that the presence of saturated fatty acids results in a decrease in the uptake of ozone by the mixed particle relative to a pure oleic acid particle. To further evaluate the ability of our method to probe these changes in reactivity, we generated mixed oleic acid-palmitic acid aerosols in ratios of 1.0, 0.8, 0.5, 0.2 and 0.0 through homogeneous nucleation. Ozonolysis of the multi-component aerosol as a function of ozone exposure similarly showed a decrease in the extent of reaction with an increase in the fraction of saturated fatty acid present in the aerosol (Figure 3).
The second project, heterogeneous chemistry of multi-component aerosols, has provided some very exciting preliminary results. Oleic acid aerosols were generated using the pneumatic nebulizer and reacted with different concentrations of ozone at a constant residence time in the flow cell. A recent review of studies of this heterogeneous reaction system indicates that observed product species include the primary organic acid and aldehyde products with additional evidence of products from the secondary chemistry of the Criegee intermediates. One of the first challenges faced in studying the ozonolysis of oleic acid with the AFT-FTIR, developed as part of this proposal, is that oleic acid contains a carboxylic acid functional group prior to ozonolysis (Figure 1) and could the aldehydes and products from the secondary chemistry be distinguished from the initial carboxylic acid absorption feature? Absorption features attributed to aldehyde and ester functionalities are observed indicating the presence of primary and secondary products (Figure 2). Integration of the carbonyl region, normalized to the initial absorbance, demonstrates a linear increase in the extent of reaction as a function of ozone exposure. Other studies have also shown that the presence of saturated fatty acids results in a decrease in the uptake of ozone by the mixed particle relative to a pure oleic acid particle. To further evaluate the ability of our method to probe these changes in reactivity, we generated mixed oleic acid-palmitic acid aerosols in ratios of 1.0, 0.8, 0.5, 0.2 and 0.0 through homogeneous nucleation. Ozonolysis of the multi-component aerosol as a function of ozone exposure similarly showed a decrease in the extent of reaction with an increase in the fraction of saturated fatty acid present in the aerosol (Figure 3).
 Finally, we spent some portion of the summer working on the infrared spectral characterization of humic like substances (HULIS) in atmospheric particulate matter. HULIS is that fraction of atmospheric aerosols that resembles terrestrial and aquatic humic substances. This group of high molecular weight compounds is thought to be responsible for much of the cloud condensation nucleation (CCN) ability of atmospheric aerosols. This project relates to our heterogeneous studies as it is currently believed that ozonolysis of unsaturated fatty acids which forms peroxides plays a role in HULIS formation in the troposphere. As the fundamental interest lies in the water soluble fraction, we collected aerosols generated through biomass combustion of cellulose fiber filters, extracted with deionized water, performed solid phase extraction of neutral and acidic pH fractions followed by elution with methanol and analysis using attenuated total reflectance FTIR. In comparison of HULIS with similar extracts from IHSS sediment samples and Fluka Humic Acid, we found that HULIS has similar functional group characteristics in corresponding fractions to these other substances.
Finally, we spent some portion of the summer working on the infrared spectral characterization of humic like substances (HULIS) in atmospheric particulate matter. HULIS is that fraction of atmospheric aerosols that resembles terrestrial and aquatic humic substances. This group of high molecular weight compounds is thought to be responsible for much of the cloud condensation nucleation (CCN) ability of atmospheric aerosols. This project relates to our heterogeneous studies as it is currently believed that ozonolysis of unsaturated fatty acids which forms peroxides plays a role in HULIS formation in the troposphere. As the fundamental interest lies in the water soluble fraction, we collected aerosols generated through biomass combustion of cellulose fiber filters, extracted with deionized water, performed solid phase extraction of neutral and acidic pH fractions followed by elution with methanol and analysis using attenuated total reflectance FTIR. In comparison of HULIS with similar extracts from IHSS sediment samples and Fluka Humic Acid, we found that HULIS has similar functional group characteristics in corresponding fractions to these other substances.
During this past funding period, five students have worked on projects supported by this grant, with four of the five having prepared a poster for presentation during an on-campus poster session. They have gained experience with method development, characterization, validation and calibration. They are proficient at generating aerosols using pneumatic nebulizers and homogeneous nucleation methods. They have performed atmospheric compensation, baseline correction, spectral averaging, data exporting, normalization, curve fitting and integration as well as explored trends and identified spectral absorption features. The faculty stipend allowed me to spend valuable dedicated time mentoring these students and focusing on the intricacies of the projects without outside distraction. The support provided by this grant has been instrumental in establishing my laboratory and developing this method for use in current and future studies; and the undergraduate research experiences provided and pending manuscripts are critical components to my success at Davidson College.