
ACS PRF | ACS
All e-Annual Reports

43705-G1
Total Synthesis of Kinamycin Antibiotics
Figure 1. Synthetic targets

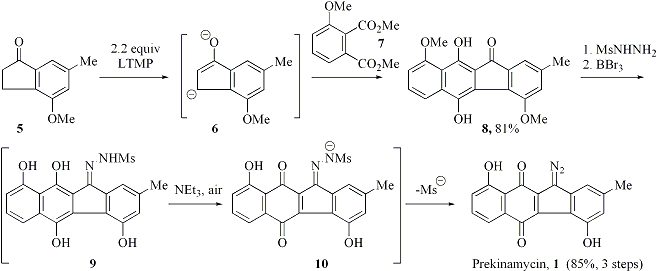
The goal of this project is to develop concise synthetic routes to the 11-diazobenzo[b]fluorene family of antibiotics, such as prekinamycin 1, kinamycins (e.g., 2), FL-120 B 3 and, ultimately, lomaiviticins (e.g., 4), which possess potent antibacterial and anticancer activities. In the course of our study, we have developed a more convenient approach to the construction of the tetracyclic core of these compounds than the one described in the original proposal. We have discovered that treatment of indanone dianions (6) with phthalate diesters (7) leads to formation of benzo[b]fluorenones (8) in good yields and virtually complete regioselectivity. In addition, we have devised an efficient protocol for the installation of the diazo group present in the above natural products. Taking advantage of these two new methods, we have accomplished a concise synthesis of prekinamycin 1 illustrated in Scheme 1.
Scheme 1. Synthesis of prekinamycin
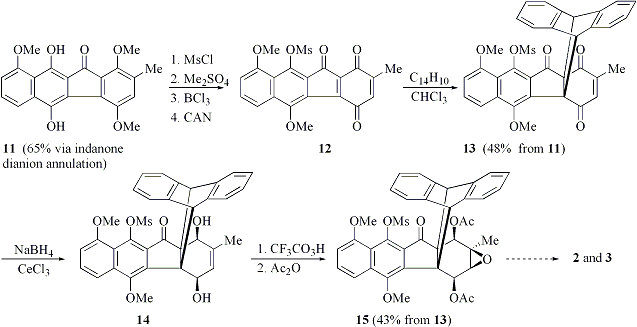
We have also achieved significant progress en route to kinamycin C and Fl-120B (Scheme 2). Starting from benzofluorenone 11 available from the indanone dianion annulation, we obtained quinone 12, which underwent regioselective Diels-Alder reaction with anthracene under mild conditions. The 9,10-dihydroanthracene moiety in 13 was designed to serve a dual purpose: to block the internal double bond of the quinone moiety and to introduce the steric bias necessary for elaboration of the stereochemically complex ring D. Stereoselective borohydride reduction of 13 followed by syn-epoxidation and acetylation gave rise to epoxydiol diacetate 15, in which the four contiguous stereocenters correspond to those in FL-120B. We have shown that regioselective epoxide opening leading to the relative stereochemistry of kinamycin C can be achieved under basic conditions. Our ongoing efforts are focused on achieving the retro-Diels-Alder reaction. Subsequent deprotection and installation of the diazo group is expected to complete the syntheses of the target compounds.
Scheme 2. Progress towards kinamycin C and FL-120B
The financial support provided by the Petroleum Research Fund has made possible the synthetic studies outlined above, which, in turn, have led to our ongoing work in related directions, including studies towards lomaiviticins and enantioselective oxidation of phenols. Thus, ACS-PRF has made an important contribution to establishing an active research program within our group.