
ACS PRF | ACS
All e-Annual Reports

44099-GB6
Measuring Uptake Coefficients for Polycyclic Aromatic Hydrocarbons onto Aerosol Particles Using Photoionization Detection
Our research group has been active in characterizing the surface polarity and morphology of model tropospheric aerosol particles using probe molecule spectroscopy. A recent result of this work that was supported by the Petroleum Research Fund involves the surface morphology and phase behavior of model tropospheric particles. We study internally mixed aerosols made from salts (NaCl, KI) and the surfactant, sodium dodecyl sulfate (SDS), as models for particles of marine origin. At high values of relative humidity (RH), these particles exists as reverse micelles (aqueous salt core surrounded by a coating of the surfactant molecules), while at low RH, they comprise a solid core of salt coated with SDS and water. By measuring the photoelectric charging efficiency and the electronic spectroscopy of our probe molecule, coumarin 314 (C314), we detect two separate phase transitions in going from high to low RH. The first transition is the efflorescence of the aqueous salt core (45% RH for NaCl/SDS and 38% RH for KI/SDS), and this behavior is very similar to that for salt particles without a surfactant coating. Following this phase transition, a thin film of water and SDS remains on the surface. This thin film undergoes efflorescence at 5% RH. We also observe a hysteresis effect associated with this low RH phase transition such that the surface morphology of these particles depends on it history over a wide range of RH. Lastly, we find that this low RH phase transition is absent when the SDS mass percent (with respect to the dry particle) is below 3%. Using this threshold, we calculate the surface coverage of SDS must approach the saturated monolayer (~ 40 Å2 head group area) for the thin film to form.
In more recent work, we use this characterization of surfactant coated particles to understand trends in the rate of uptake of polycyclic aromatic hydrocarbons (PAHs) onto 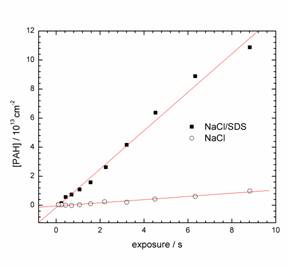 the particles' surface. In this experiment, the aerosol particle stream interacts with a known pressure of PAH in a sliding injector flow tube. We monitor the surface concentration of PAH as a function of particle-PAH interaction time using laser photoionization of the surface-bound PAH. From the resulting surface concentration vs. exposure time plot, we can determine the initial uptake coefficient, g, which is the initial uptake rate normalized to the gas-particle collision rate. The figure at the right shows two representative uptake curves, corrected for first-order PAH loss on the walls of the flow tube. The preliminary results show that the uptake rate of pyrene onto SDS-coated NaCl aerosol particles (g ~ 1.1x10-3) is roughly a factor of 10 larger than that for the pure NaCl particles. Clearly, the SDS coating enhances the uptake rate. We are currently investigating the effects of the various morphology changes associated with these internally mixed particles on the uptake rates.
the particles' surface. In this experiment, the aerosol particle stream interacts with a known pressure of PAH in a sliding injector flow tube. We monitor the surface concentration of PAH as a function of particle-PAH interaction time using laser photoionization of the surface-bound PAH. From the resulting surface concentration vs. exposure time plot, we can determine the initial uptake coefficient, g, which is the initial uptake rate normalized to the gas-particle collision rate. The figure at the right shows two representative uptake curves, corrected for first-order PAH loss on the walls of the flow tube. The preliminary results show that the uptake rate of pyrene onto SDS-coated NaCl aerosol particles (g ~ 1.1x10-3) is roughly a factor of 10 larger than that for the pure NaCl particles. Clearly, the SDS coating enhances the uptake rate. We are currently investigating the effects of the various morphology changes associated with these internally mixed particles on the uptake rates.