
ACS PRF | ACS
All e-Annual Reports

44989-B1
Stereochemical Studies of Fluorescent Troger's Bases
The proposed approach to the synthesis of the monomeric precursors of the required fluorescent Tršger's bases required sequential formylation of an N-alkyl-4-amino-1,8-naphthalimide, Grignard addition to the aldehyde, and optical resolution of the resultant secondary alcohol.

The direct synthesis of the Tršger's bases by treating the aminonaphthalimide with acetaldehyde and hydrochloric acid under the standard conditions has failed to give any identifiable products at this time, but we are continuing to study this reaction as a potential method for the synthesis of the required compounds. Impact on the P.I. and students The grant has provided much needed support to move our work in the area of fluorescent compounds forward, and has resulted in the discovery of heretofore unreported reactivity in the naphthalimide system. We expect this to be a fertile area of research for the future, and to extend this line of research beyond the end of this grant. Four students have been directly involved in this project for some period during the reporting period. Leah Groess and Ashley Dreis graduated with B.S. degrees in chemistry, and are pursuing graduate study at the University of Minnesota. Justin Ryback graduated with a B.S. in chemistry, and is spending a year working in industry before returning to graduate school. Elizabeth Raupach continues working on the project, and is now a junior. Kelsey Dunkle is a senior has been working on the project since January, 2007, and will continue to do so this year. She will move to being funded by the grant in the Fall semester. Kyle Kopidlansky is a junior who has been working peripherally on the project, and who will move onto the project more directly during this year. Gina Macek is a senior who is working on a related project, and on an unrelated project. 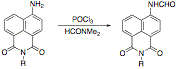 To date, we have found that, despite relatively facile bromination at position 3 of the naphthalimide nucleus, acylation and hydroxyalkylation in general have been elusive reactions to accomplish. The treatment of 4-amino-N-hexyl-1,8-naphthalimide under standard Vilsmeier-Haack reaction conditions resulted in the formation of the N-arylformamide instead of the anticipated aldehyde.
To date, we have found that, despite relatively facile bromination at position 3 of the naphthalimide nucleus, acylation and hydroxyalkylation in general have been elusive reactions to accomplish. The treatment of 4-amino-N-hexyl-1,8-naphthalimide under standard Vilsmeier-Haack reaction conditions resulted in the formation of the N-arylformamide instead of the anticipated aldehyde. 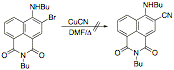 In a effort to circumvent this problem, we attempted the displacement of bromide from 3-bromo-4,N-dibutyl-1,8-naphthalimide with cuprous cyanide under Ullmann conditions, reasoning that we could then modify the nitrile by reduction or Grignard addition. The displacement fails in our hands. We are about to investigate the Sonogashira coupling of trimethylsilylacetylene with the bromide in an effort to provide a precursor for the 3-acetyl compound.
In a effort to circumvent this problem, we attempted the displacement of bromide from 3-bromo-4,N-dibutyl-1,8-naphthalimide with cuprous cyanide under Ullmann conditions, reasoning that we could then modify the nitrile by reduction or Grignard addition. The displacement fails in our hands. We are about to investigate the Sonogashira coupling of trimethylsilylacetylene with the bromide in an effort to provide a precursor for the 3-acetyl compound.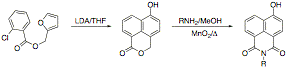 As an alternative approach, we have begun to investigate the synthesis of the 4-hydroxy-1,8-naphthalimide by intramolecular benzyne cycloaddition to furans. The endoxide formed in this reaction we expect to react with base to regenerate the aromatic naphthol, and we then expect to be able to effect aminolysis of the lactone in the presence of manganese dioxide to oxidize the revealed benzylic alcohol through the aldehyde to the imide.
As an alternative approach, we have begun to investigate the synthesis of the 4-hydroxy-1,8-naphthalimide by intramolecular benzyne cycloaddition to furans. The endoxide formed in this reaction we expect to react with base to regenerate the aromatic naphthol, and we then expect to be able to effect aminolysis of the lactone in the presence of manganese dioxide to oxidize the revealed benzylic alcohol through the aldehyde to the imide.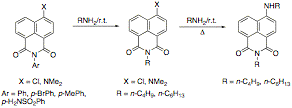 As part of the exploratory work in naphthalimide chemistry supported in part by this grant, we have discovered new, unexpected reactivity in the naphthalimide nucleus. Two manuscripts are now in preparation describing these new findings. Specifically, we have found that the naphthalimide ring of 4-substituted-N-aryl-1,8-naphthalimides is much more susceptible to attack by nucleophiles (especially primary amines) in direct contradiction of over six decades of conventional wisdom and experimental reports. We have found that the displacement of the N-aryl group is effected by primary alkylamine nucleophiles in the absence of solvent, and that this displacement is not dependent on either the substituent at the 4- position of the ring, or on the substitution of the aromatic ring of the N-aryl substituent. Interestingly, only the N-aryl compounds exhibit this reactivity. We have found no evidence for the exchange of an N-alkyl group during displacement of the 4-chloro group by amine nucleophiles either at reflux or at room temperature.
As part of the exploratory work in naphthalimide chemistry supported in part by this grant, we have discovered new, unexpected reactivity in the naphthalimide nucleus. Two manuscripts are now in preparation describing these new findings. Specifically, we have found that the naphthalimide ring of 4-substituted-N-aryl-1,8-naphthalimides is much more susceptible to attack by nucleophiles (especially primary amines) in direct contradiction of over six decades of conventional wisdom and experimental reports. We have found that the displacement of the N-aryl group is effected by primary alkylamine nucleophiles in the absence of solvent, and that this displacement is not dependent on either the substituent at the 4- position of the ring, or on the substitution of the aromatic ring of the N-aryl substituent. Interestingly, only the N-aryl compounds exhibit this reactivity. We have found no evidence for the exchange of an N-alkyl group during displacement of the 4-chloro group by amine nucleophiles either at reflux or at room temperature. During related work, peripherally supported by PRF, we have discovered an apparently unprecedented reaction of N-allyl-4-alkylamino-1,8-naphthalimides with bromine in aprotic solvents. In this reaction, we find that the carbonyl oxygens participate in the reaction, with the intermediate cation being not the bromionium ion, but an iminium ion that is attacked at position 3 of the ring to return a product in which ring bromination has occurred in preference to simple addition to an allyl group. We have been able to isolate the intermediate iminium ion and characterize it by 1H NMR spectroscopy. In protic solvents, the bromination proceeds as anticipated to give addition to the allyl group, which suggests that the major product of the reaction is determined by the relative locations of the ions involved: solvent-separated ion pairs give the "normal" addition product, while intimate ion pairs give the ring-brominated product.
During related work, peripherally supported by PRF, we have discovered an apparently unprecedented reaction of N-allyl-4-alkylamino-1,8-naphthalimides with bromine in aprotic solvents. In this reaction, we find that the carbonyl oxygens participate in the reaction, with the intermediate cation being not the bromionium ion, but an iminium ion that is attacked at position 3 of the ring to return a product in which ring bromination has occurred in preference to simple addition to an allyl group. We have been able to isolate the intermediate iminium ion and characterize it by 1H NMR spectroscopy. In protic solvents, the bromination proceeds as anticipated to give addition to the allyl group, which suggests that the major product of the reaction is determined by the relative locations of the ions involved: solvent-separated ion pairs give the "normal" addition product, while intimate ion pairs give the ring-brominated product.