
ACS PRF | ACS
All e-Annual Reports

45324-GB1
Synthesis of Thionitrites Using Electrogenerated Cobalt(I) Salen as the Mediator
Most commonly used methods for the synthesis of thionitrites (RSNO) have disadvantages. We proposed a simple route with mild synthetic conditions to make the compounds by using electrogenerated cobalt(I) salen as the mediator, as shown below:
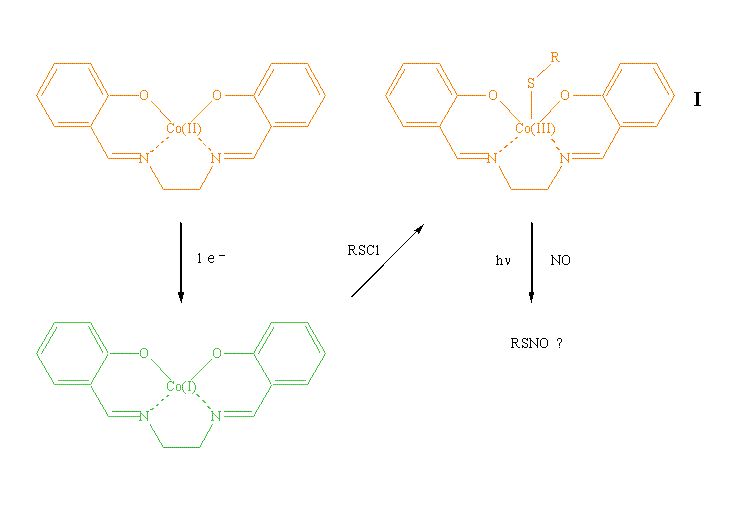
The formation of the thiylcobalt(III) salen complex (I) is the most significant step for the generation of thionitrites. Various sulfenyl chlorides (RSCl) will be used in this project to test the efficiency of the method. We have synthesized a series of aromatic sulfenyl chlorides as the substrates according to a reported procedure.1 These compounds were purified by vacuum distillation and characterized by 1H NMR (400 MHz, CDCl3): (a) for benzenesulfenyl chloride, dH (ppm) 7.42 (3 H, m, Ph), 7.67 (2 H, m, Ph); (b) for 4-methylbenzenesulfenyl chloride, dH (ppm) 2.40 (3 H, s, CH3), 7.24 (2 H, d, Ph), 7.62 (2 H, d, Ph); and (c) for 4-chlorobenzenesulfenyl chloride, dH (ppm) 7.38 (2 H, d, Ph), 7.60 (2 H, d, Ph).
Cyclic voltammograms (CV) were collected for five aromatic sulfenyl chlorides including 2-nitro and 4-nitrobenzenesulfenyl chlorides, which are commercially available. Figure 1 depicts the CVs recorded with a 3-mm diameter glassy carbon electrode at 200 mV s–1 in acetonitrile containing 0.050 M tetramethylammonium tetrafluoroborate (TMABF4). It can be observed that the reduction of sulfur–chlorine bonds takes place at relatively positive potentials for all the compounds, as summarized in Table 1. In fact, it is much more easy for the cleavage of the sulfur–chlorine bond than for the one-electron reversible reduction of the cobalt(II) salen–cobalt(I) salen couple (–1.32 V vs. SCE).2 Consequently, it is not feasible to electrochemically reduce cobalt(II) salen in the presence of sulfenyl chlorides to generate the thiylcobalt(III) salen intermediates (I). Instead, we first reduced cobalt(II) salen to cobalt(I) salen in acetonitrile at –1.40 V vs. SCE. The color of the solution turned from orange to dark green. Under inert conditions, the substrates were added and the color of the solution immediately turned to yellow-orange, indicating that the reaction of electrogenerated cobalt(I) salen and sulfenyl chlorides took place. Cyclic voltammetric features of the resulting solution showed the evidence for the formation of complex I. The species is currently under UV-Vis spectroscopic examination for further confirmation.3 After the thiylcobalt(III) salen (I) is characterized, nitric oxide (NO) will be infused into the solutions containing I that are simultaneously illuminated with various light sources. It is expected that thionitrites will be obtained as the products, which are subject to complete characterization and quantification. Our ultimate goal is to find the optimal experimental conditions for the synthesis. The outcome of the project will serve as the foundation for the development of a methodology that is applicable to the general conversion of various organic halides and NO to useful products.
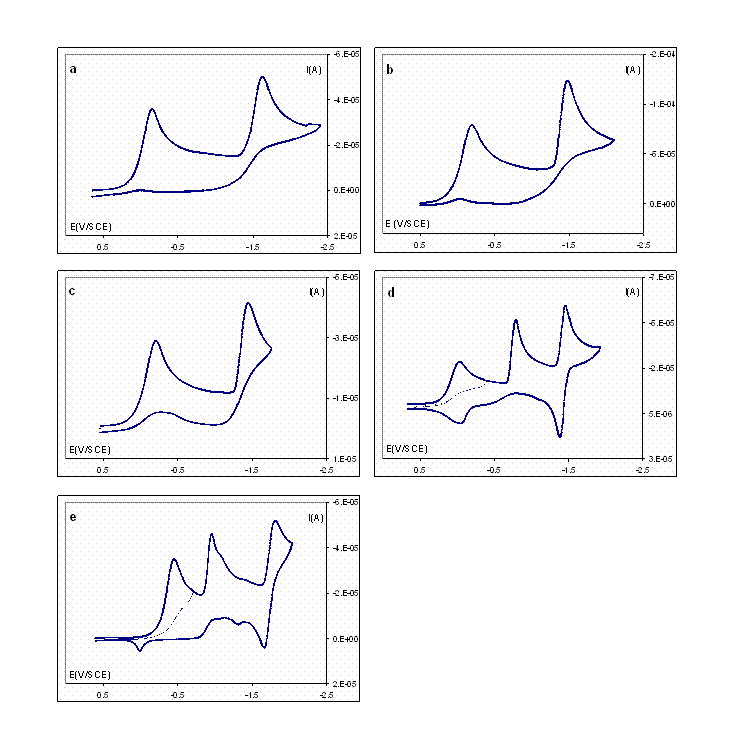
Figure 1. Cyclic voltammograms recorded with a glassy carbon electrode at 200 mV s–1 in acetonitrile containing 0.050 M TMABF4 and 2 mM (a) 4-methylbenzenesulfenyl chloride, (b) benzenesulfenyl chloride, (c) 4-chlorobenzenesulfenyl chloride, (d) 4-nitrobenzenesulfenyl chloride, and (e) 2-nitrobenzenesulfenyl chloride.
Table 1. Electrochemical reduction potentials (vs. SCE) of aromatic sulfenyl chlorides.
Compound | 4-MePhSCl | PhSCl | 4-ClPhSCl | 4-NO2PhSCl | 2-NO2PhSCl |
Ep, V | –0.18 | –0.21 | –0.22 | –0.05 | –0.46 |
Three undergraduate students participated in this project during the past year. Trained by the principal investigator (PI), the students have gained extensive experience in organic synthesis, product purification and characterization, as well as spectroscopic analysis. They have also learned various important electrochemical techniques such as cyclic voltammetry and controlled-potential bulk electrolysis. The project inspired the students to get more interested in chemistry research. Two students received B.S. degree in May 2007 and were admitted to graduate school (William C. Silvers – Ph.D. program at Louisiana State University, Susanne M. Boisvert – M.S. program at Texas State University).
The project also has a profound impact on the career of the PI, who is a tenure-track assistant professor at Texas State University. By working with the students in the laboratory, the PI continued to accumulate his experience in undergraduate research mentoring. Several proposals which are related to this project have since been submitted. The study has put his research on track and may potentially lead to a productive program.
References
1. Harpp, D. N.; Friedlander, B. T.; Smith, R. A. Synthesis 1979, 3, 181.
2. Alleman, K. S.; Samide, M. J.; Peters, D. G.; Mubarak, M. S. Current Topics in Electrochemistry 1998, 6, 1.
3. Klein, L. J.; Alleman, K. S.; Peters, D. G.; Karty, J. A.; Reilly, J. P. J. Electroanal. Chem. 2000, 481, 24.