
ACS PRF | ACS
All e-Annual Reports

44904-AC3
Rigid Pincer Ligands: New Prospects in Hypercoordinate Main Group Chemistry
Grant Report for the ACS PRF award #44904-AC3.
September 2006 – August 2007.
Hypercoordinate Al complexes.1 Our investigations focused on the synthesis of five-coordinate Al complexes supported by the rigid PNP pincer ligands and the feasibility of the formation of a hypercoordinate Al alkylidenes. Preparation of various (PNP)AlX2 complexes 2-4 was successfully accomplished via alkane elimination reactions of EtAlCl2 or trialkylaluminums with (PNP)H. The structure of (PNP)AlCl2 (2) was determined by X-ray diffractometry and was shown to contain a five-coordinate Al center in an approximately trigonal-bipyramidal geometry. The structures of (PNP)AlCl2 (2), (PNP)AlMe2 (3), and (PNP)AlBui2 (4) were probed by DFT computational methods (in collaboration with Prof. D. Yandulov). DFT studies show that the five-coordinate structure is preferred for all three compounds, although the preference decreases in the substituent order of Cl > Me > Bui. Computations were also performed to investigate whether a-abstraction from 3* to produce an aluminum alkylidene 5* is thermodynamically favorable. The findings indicate that such a reaction is decidedly unfavorable. It is unlikely that the high endoergonics of this transformation may be overcome by varying the Al-bound alkyl groups.
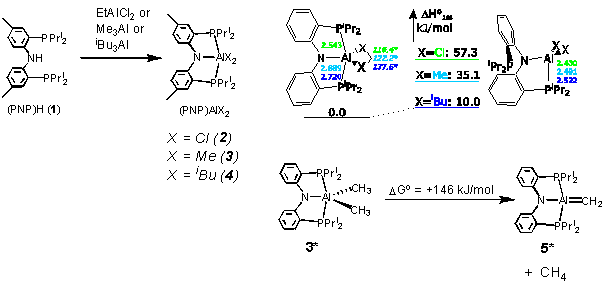 Figure 1. Synthesis and DFT studies of PNP complexes of Al.
Figure 1. Synthesis and DFT studies of PNP complexes of Al.
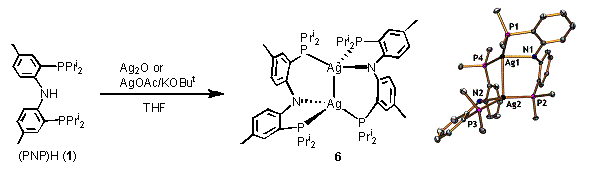 Dimeric PNP complex of silver(I).2 In the course of our studies, we prepared a Ag(I) derivative of the PNP ligand. We surmised that a (PNP)Ag complex may serve as a convenient PNP transfer agent. [(PNP)Ag]2 (6) exists as a dimer both in solution and in the solid state and can be conveniently prepared from simple Ag(I) starting materials and (PNP)H. [(PNP)Ag]2 (6) is an air-, moisture-stable compound (unlike (PNP)Li or (PNP)Tl) and serves well to transfer the PNP ligand to divalent group 10 metals. It is worth noting that both the Al and the Ag chemistry was performed primarily by a talented undergraduate Jessica DeMott who is presently a Ph.D. student in chemistry at Stanford.
Dimeric PNP complex of silver(I).2 In the course of our studies, we prepared a Ag(I) derivative of the PNP ligand. We surmised that a (PNP)Ag complex may serve as a convenient PNP transfer agent. [(PNP)Ag]2 (6) exists as a dimer both in solution and in the solid state and can be conveniently prepared from simple Ag(I) starting materials and (PNP)H. [(PNP)Ag]2 (6) is an air-, moisture-stable compound (unlike (PNP)Li or (PNP)Tl) and serves well to transfer the PNP ligand to divalent group 10 metals. It is worth noting that both the Al and the Ag chemistry was performed primarily by a talented undergraduate Jessica DeMott who is presently a Ph.D. student in chemistry at Stanford.
Figure 2. Synthesis and structure of [(PNP)Ag]2 (6).
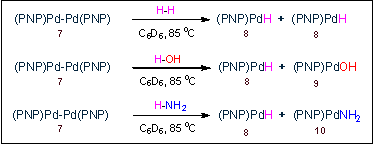 Activation of dihydrogen, water, and ammonia by a dipalladium complex.3 We have isolated unusual PdI-PdI dimer 7 that is formed in the photochemical decomposition of (PNP)PdEt. Curiously, this is a complex of a general formula [(PNP)M]2, but has a structure that is quite different from that of [(PNP)Ag]2 (vide supra).2 We anticipated that the reactivity of this dimer will be driven by the formation of monomeric (PNP)Pd-X compounds. This has proven to be true in the reactions with very simple molecules. 7 cleanly reacts with H-H, H-OH, and H-NH2 to add the H-X bonds in these molecules across the Pd-Pd bond. This binuclear oxidative addition4 is especially remarkable for NH3. Only recently was the first well-defined example of oxidative addition of ammonia been documented5 and ours is the only example where ammonia is split into a terminal hydride and a terminal amido moiety by two metal centers.
Activation of dihydrogen, water, and ammonia by a dipalladium complex.3 We have isolated unusual PdI-PdI dimer 7 that is formed in the photochemical decomposition of (PNP)PdEt. Curiously, this is a complex of a general formula [(PNP)M]2, but has a structure that is quite different from that of [(PNP)Ag]2 (vide supra).2 We anticipated that the reactivity of this dimer will be driven by the formation of monomeric (PNP)Pd-X compounds. This has proven to be true in the reactions with very simple molecules. 7 cleanly reacts with H-H, H-OH, and H-NH2 to add the H-X bonds in these molecules across the Pd-Pd bond. This binuclear oxidative addition4 is especially remarkable for NH3. Only recently was the first well-defined example of oxidative addition of ammonia been documented5 and ours is the only example where ammonia is split into a terminal hydride and a terminal amido moiety by two metal centers.
References.
(1) DeMott, J. C.; Guo, C.; Foxman, B. M.; Yandulov, D. V.; Ozerov, O. V. Mendeleev Commun. 2007, 17, 63 (invited contribution to the Higher Chemical College issue).
(2) DeMott, J. C.; Basuli, F.; Kilgore, U. J.; Foxman, B. M.; Huffman, J. C.; Ozerov, O. V., Mindiola, D. J. Inorg. Chem. 2007, 46, 6271.
(3) Fafard, C. M.; Adhikari, D.; Foxman, B. M.; Mindiola, D. J.; Ozerov, O. V. J. Am. Chem. Soc. 2007, 129, 10318.
(4) Crabtree, R. H. The Organometallic Chemistry of the Transition Metals, 4th ed.; Wiley-Interscience: New York, 2005, pp. 160-161.
(5) Zhao, J.; Goldman A. S.; Hartwig J. F. Science, 2005, 307, 1080.