ACS PRF | ACS
All e-Annual Reports

42447-G7
A Coarse Grained Model for Studying Inhomogeneous Membrane Interfaces
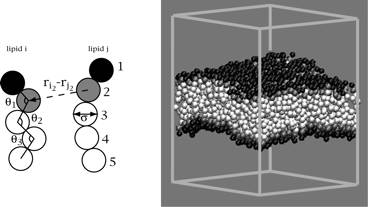
We have developed a novel solvent-free model for lipid molecules, which is appropriate for modeling lipid bilayers at a coarse-grained level of resolution. Within the model, lipids are considered as a semi-flexible chain of five beads (Fig. 1).
Figure SEQ Figure \* ARABIC 1: Lipids are represented by chains of five beads. Head beads are shown in black, tail beads in white and interface beads in gray. A self-assembled bilayer composed of 800 lipid molecules is shown on the right Head beads (shown in black) represent the polar lipid heads and interact via a purely repulsive interaction potential. Tail beads (shown in white) represent the oily lipid tails and interact via a standard 6-12 Lennard-Jones potential. We mimic the hydrophobic effect at the interface between lipid head and tail beads to achieve bilayer stability. "Interfacial" beads (shown in gray) interact through an attractive, but unusually flat, potential that is parameterized to correctly reproduce the interfacial tension present in real bilayer systems. The lipids described above interact with one another in the absence of any explicit representation of water. The hydrophobic effect is captured implicitly through the attractive interaction of interfacial beads in the system. From a computational standpoint, this model is very appealing. Removing solvent from explicit consideration allows us to run simulations with many fewer particles than is typically necessary and makes feasible simulations that would be prohibitively expensive with traditional solvated models. The simplified lipids behave in accord with the expected properties of experimental membrane systems: the lipids self assemble, the elastic properties of the bilayer (bending modulus, compression modulus) lie in the range of experimental systems, the stress profile of the bilayer agrees with results from more elaborate Molecular Dynamics simulations and the interfacial tension measured in the system agrees with experimental and theoretical estimates. At present this model is appropriate to study thermodynamic properties via Monte Carlo simulation, but not system dynamics. We have applied this model to study membrane deformations caused by the presence of integral membrane proteins in the lipid bilayer. Membrane bound proteins typically display hydrophobic regions at least somewhat incommensurate with the thickness of the bilayer that surrounds them. This "hydrophobic mismatch" must be somehow compensated for in a composite protein-bilayer system. (It would be energetically prohibitive to leave hydrophobic regions directly exposed to water.) Various analytical theories have been proposed to explain how the bilayer adjusts to the presence of a protein. Until recently, it has not been possible to address this question via simulations. Fully atomic studies are too expensive to allow simulations over the necessary time and length scales to achieve meaningful results and even coarse-grained models that include solvent have been run in geometries too small to insure the absence of periodic boundary condition artifacts. We have run multiple simulations of membrane bound proteins within large bilayer environments. An example is displayed in Fig. 2. Figure SEQ Figure \* ARABIC 2: Bilayer with embedded membrane protein. The protein has a somewhat larger hydrophobic region that the thickness of the homogeneous bilayer and this leads to the splaying open of the bilayer in the immediate vicinity of the protein. Our results have led to the first critical analysis of elasticity theory in the context of protein-induced bilayer deformations. Interestingly, even at the seemingly microscopic level of the observed deformations we find that elastic models work exceedingly well. Our studies have actually guided our development of the first elastic model that is completely consistent with the available experimental and simulation data available in the literature (and in our own simulations). We find that it is essential to include the possibility of finite monolayer spontaneous curvature and saddle-splay modulus to achieve correspondence with simulations. This fact is not widely appreciated in the literature. Our studies have highlighted one of the very important roles for coarse-grained modeling in biophysics. Coarse-grained models can be used to validate and/or refute hypothetical analytical models. We find this approach very rewarding. The coarse-grained lipid models studied under our PRF funding have suggested numerous avenues to explore theoretically and experimentally. The research carried out under this grant has had a substantial positive impact upon the career of my students and myself. I have recently been awarded tenure at UC Santa Barbara and the work carried out under the auspices of this ACS-PRF grant undoubtedly played a role in this decision by the University. The primary graduate student involved in this project, Grace Brannigan, mastered a variety of theoretical/computational tools and skills during the course of her research, which will server her well in her future scientific career. In addition, two undergraduates and another graduate student played minor roles in this project. While their efforts did not ultimately result in publishable research, they did gain valuable experience that will serve them well in future research projects.