www.acsprf.org
Reports: DNI1050283-DNI10: Atomistic Simulations of the Chemistry of Environmentally Assisted Cracking in Nickel-Chromium Alloys
Srinivasan Srivilliputhur, PhD , University of North Texas
SUMMARY: The project is on track. Two PhD students currently work on this project. The project started in January 2011 instead of 09/2010 because PhD student Ms. Priyanvada Paranjape's visa paperwork got delayed. Priyanvada has a Masters degree in Chemical Engineering (specialization in corrosion). She started the computational work in 03/2010 while we added an experimental component to the embrittlement work in 01/2010 with Mr. Ravi Rajamure (MS from Cleveland State University). Both computational and experimental work is progressing very rapidly (despite a delayed start).
Ravi finished the first part of his work in developing coating using laser surface engineering methods to resist corrosion. First draft of his manuscript is complete and will be submitted in March 2012 to the Journal Corrosion Science. Ravi is also writing a review paper on the state of knowledge on embrittlement. He will take the PhD qualifier exam in 05/2012.
Priyanvada spent considerable time learning computational modeling (large scale parallel molecular dynamics and first principles density functional theory (DFT) methods). She is currently carrying out DFT calculation of hydrogen diffusion in nickel-chromium (Ni-Cr) system and has made considerable progress. She also passed the PhD qualifying exams in 05/2011.
Below, we present DFT results on hydrogen effects in Ni and Ni-Cr.
Introduction:
Environmentally assisted cracking2 (EAC) is a general term that includes stress corrosion cracking (SCC) and hydrogen embrittlement (HE). EAC is a phenomenon by which a normally ductile metal looses its toughness when it is subjected to mechanical stresses in presence of a corroding environment. Localized cracks rapidly form and catastrophically propagate due to synergy between a susceptible material, tensile stresses, and material's environment. Certain events like surface diffusion, surface reactions, absorption into bulk, bulk diffusion to the plastic zone ahead of the advancing crack, chemical reactions in the bulk, and the rate of interatomic bond rupture should occur for sustained crack propagation.
Diffusion plays an important role in understanding the properties of materials like phase transformations, segregation, hydrogen embrittlement and corrosion. In the present work the migration energy barriers for hydrogen in pure Ni and ordered Ni2Cr have been calculated. The migration barriers quantifies barrier to H atom diffusion in the bulk lattice of Ni and Ni2Cr alloy. We found that pure Ni is more susceptible to embrittlement as compared to Ni alloyed with Cr.
Simulation Methodology and Results:
DFT has been used to find out the ground state energy of relaxed structures in Ni-H and Ni2Cr-H system. Nudged elastic band (NEB) method is used to find out the migration energy barrier for H atom.
Ni-H and Ni-Cr-H systems: Ground state energy of Ni and Ni2Cr structures was found by including and excluding magnetic effects in our calculations and are tabulated in Table-1. These calculations are performed for system containing 32 atoms of Ni and one H impurity at tetrahedral and octahedral sites. Plane wave cut off energy of 450eV is used for geometry optimization and evaluation of ground state energies. Convergence calculations were systematically done to quantify for system size effects. Methfessel–Paxton smearing with a width of 0.2eV.
Hydrogen atom prefers octahedral interstitial site to tetrahedral site in Ni lattice because of lower energy for octahedral site occupancy, also shown by Extended X-Ray Absorption Fine Structure (EXAFS).3 Magnetism effects were included in the NEB calculations.
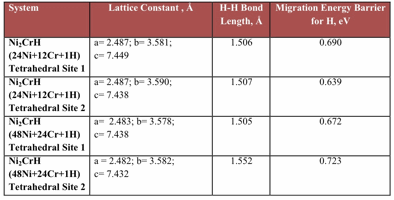
Ground state energies of relaxed structures of Ni2Cr-H system are shown in table 2. These calculations are performed for system containing 32 host atoms of Ni, 16 atoms of Cr and one H impurity. There are two tetrahedral interstitial sites for H atom in Ni2Cr lattice. 3 Ni and 1 Cr atoms surround one tetrahedral site and 2 Ni atoms and 2 Cr atoms surround the other site. Hydrogen atom prefers to occupy octahedral interstitial site as compared to tetrahedral site in Ni2Cr lattice since the energies for octahedral site occupancy is lower than the tetrahedral site occupancy. Magnetic effects are negligible for Ni2Cr.
NEB calculations give the migration energy barrier for hydrogen atom from tetrahedral to octahedral interstitial sites. These barriers for hydrogen atom in pure Ni are shown in figure1 and 2 and tabulated in table 3. Similarly migration energy barrier for H atom in Ni2Cr are shown in figure 3 and tabulated in table 4. There are two tetrahedral sites for H atom in ordered Ni2Cr alloy.
To summarize, Bulk diffusion of H is faster in pure Ni than Ni2Cr alloy. This will ensure lower susceptibility for hydrogen embrittlement when chromium is alloyed with pure nickel.
Future Work: Our future goal is to understand embrittlement mechanisms in materials and plan to:
1. Investigate the energetics of structures by incorporating defects such as vacancies, grain boundaries, dislocations, and stacking faults.
2. Understand effect of defects on hydrogen transport during embrittlement.
3. Use kinetic Monte Carlo (KMC) method to study kinetics and thermodynamics of embrittlement over long length and time scales. Diffusion barriers obtained above using DFT is input for KMC.
4. Develop semi-empirical atomistic potential for Ni-Cr-H system
References:
1. E. Wimmer et al, "Temperature, Dependent Diffusion Coefficients from Ab Initio Computations: Hydrogen, Deuterium, and Tritium in Nickel", Physical Review B, 77, p.134305 (2008)
2. R.B. Rebak, " Environmentally Assisted Cracking of Nickel Alloys-A Review", Proceedings of 2nd International Conference on Environment-Induced Cracking of Metals, p.19, (2004)
3. B. Lengeler, " Lattice Site Location of Hydrogen by Use of Extended X-Ray Absorption Fine Structure", Physical Review Letters, 53, p.74 (1984)
Table 1: Ground State Energies for Ni-H System
 |
Table 2: Ground State Energies for Ni2Cr-H System
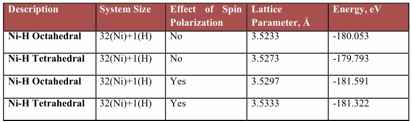 |
Table 3: Migration Energy Barrier of H in Ni-H System
 |
Table 4: Migration energy barrier for H in Ni2Cr Ordered Structure
 |
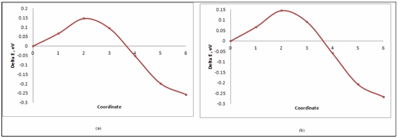 |
Figure 1: Migration Energy Barrier for pure Ni (32Ni+1H)- a) Non-magnetic system. b) Spin Polarized system
 |
Figure 2: Migration Energy Barrier for pure Ni (64Ni+1H)- a) Non-magnetic system. b) Spin Polarized system
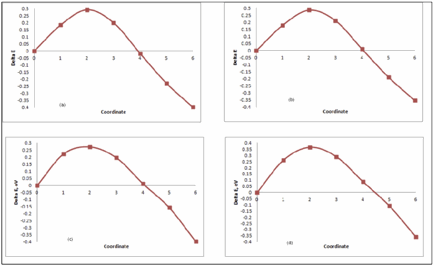 |
Figure 3: Migration Energy Barrier for Ni2Cr- (a) System (24Ni+12Cr+1H) Tetrahedral site 1. (b) System (24Ni+12Cr+1H) Tetrahedral site 2. (c) 48Ni+4Cr+1H) Tetrahedral site 1. (d) 48Ni+24Cr+1H) Tetrahedral site 2
