www.acsprf.org
Reports: DNI749249-DNI7: Benzobisazoles as modular building blocks for novel electronic materials
Malika Jeffries-EL, Ph.D , Iowa State University
Organic semiconducting materials are currently being investigated for use in applications such as field effect transistors (FET)s, organic light emitting diodes (OLED)s, and photovoltaic cells (PVC)s. These devices can benefit from the many attractive features of organic materials such as ease of processing and the ability to tune their electron properties for specific applications through chemical synthesis. Although there are many known electron-accepting moieties, the development of new building blocks is still of interest for designing materials with improved properties. The objective of this PRF-funded research project is to develop new electron-accepting conjugated polymers for use in all polymer photovoltaic cells. To accomplish this task, we have combined experimental and theoretical methods to evaluate the impact of substitution on the central benzene ring (positions 4 and 8) of benzo[1,2-d;4,5-d'] bisoxazole. Using this approach we seek to innovate layered polymer photovoltaic cells by creation new materials with tunable polymer HOMO and LUMO levels. The final goal is to develop materials with LUMO levels that are suitable for use as electron acceptors in organic photovoltaic cells.
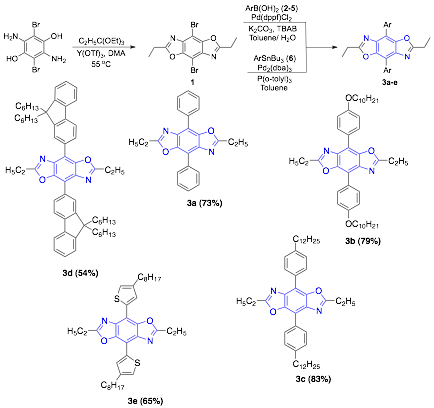
Figure 1. Synthesis of 4, 8- Substituted Benzobisoxazoles
Previously, we set out to determine which types of substituents could be attached to the central benzene ring of the benzobisoxazoles moiety to lower its LUMO level. We also sought to develop pathways for their synthesis, as the reports of such compounds are limited. Using Suzuki cross- coupling reactions we were able to extend the conjugation across position 4- and 8- by attaching substituted arenes, Figure 1. As a result of these substitutions we see a reduction in the band gap of the resulting compounds as a result of a decrease in both the LUMO and HOMO. The extent of these changes varies depending on the types of groups attached. Using this approach we are able to tune the LUMO and HOMO over a 1 eV. The experimental results show excellent correlation to the theoretical values for the HOMO levels demonstrating that we can use theory to predict and optimize the energy levels of these materials. The LUMO levels do show higher error due to the fact that the experimental measurements are done in solution. We have previously published a manuscript on alkynyl-substituted benzobisoxazoles and we are currently preparing a manuscript on these aryl-substituted derivatives.
| HOMO (eV)
| LUMO (eV)
| Egopt (eV)
| ||||||
| Experiment
| Theory
| diff
| Experiment
| Theory
| diff
| Experiment
| Theory
| diff
|
1
| -6.5 | -6.17 | 0.33 | -2.28 | -1.40 | 0.88 | 4.2 (296) | 4.44 | 0.24 |
3a
| -5.94 | -5.49 | 0.45 | -2.42 | -1.42 | 1.00 | 3.5 (294) | 3.79 | 0.29 |
3b
| -5.87 | -4.98 | 0.89 | -2.55 | -1.18 | 1.37 | 3.4 (360) | 3.50 | 0.1 |
3c
| -5.96 | -5.29 | 0.67 | -1.85 | -1.31 | 0.54 | 2.9 (403) | 3.68 | 0.68 |
3d
| -5.98 | -5.15 | 0.83 | -2.90 | -1.60 | 1.30 | 3.1 (400) | 3.21 | 0.11 |
3e
| -5.45 | -5.03 | 0.42 | -2.35 | -1.64 | 0.71 | 3.1 (269) | 3.21 | 0.11 |
Table 1. An experimental and theoretical comparison of the electronic properties of BBO model compounds
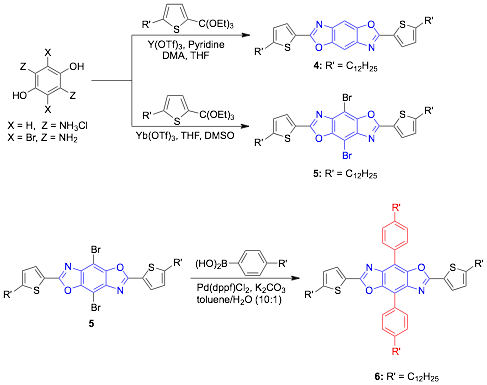
We have also synthesized two-dimensional cruciform type molecules using the synthetic strategy outlined in Figure 2. From a fundamental perspective such materials are of interest as switching out the substituents around the benzobisoxazoles can be used to stabilize the HOMO or LUMO level. In the case of these cruciforms the "parent" conjugation axis changes from through the benzobisoxazoles rings, to perpendicular to it depending on the nature of the aryl rings around the benzobisoxazole ring. This method is versatile and opens the door for the development of new tunable organic semiconductors. We are currently synthesizing a few additional examples of this class of compounds and are preparing a manuscript based on this work.
Figure 2. Synthesis of Benzobisoxazoles Cruciforms