www.acsprf.org
Reports: DNI1049045-DNI10: Metal Oxide Nanoparticles for High Energy Electrochemical Capacitors
Gleb Yushin, PhD , Georgia Institute of Technology
Supercapacitors, also called electrochemical capacitors (EC), are rechargeable electrochemical energy storage devices, which offer a much longer life cycle and higher power density than batteries. Their applications in hybrid electrical engines and power grid are growing. Transition metal-oxides exhibit fast and electrochemically-reversible Faradaic redox reactions to store charge in supercapacitors, but often suffer from low surface area and low electrical conductivity. The formation of highly conductive porous carbons decorated with inexpensive nano-sized metal oxide should circumvent the disadvantages of pure metal oxide supercapacitors. The goal for this work was to deposit transition metal oxide nanoparticles or thin films of various thickness and microstructure on carbon nanotubes and other porous carbons via vapor deposition routes and study their electrochemical properties, including specific capacitance, frequency response, power and energy density.
Atomic layer deposition (ALD) was done in a custom-built ALD system consisting of a quartz tube (heated in a furnace) through which precursor vapors were introduced alternatively. Vanadium tri-n-propoxide oxide (Gelest, Inc, USA) and de-ionized H2O (18M-Ohm) were used as precursors and were heated to ~45°C and 100°C, respectively, during the deposition. High purity Ar (99.999 %, Air Gas, USA) was used as both carrier and purging gas (residence and purging periods were 10s and 20s, respectively for the H2O precursor and for the vanadium precursor). All the precursor gas lines were maintained at 100°C during the deposition process. The pressure of the system was maintained at 4 Torr throughout the deposition.
The MWCNT electrodes used as substrates for the ALD deposition were produced as follows: 25mg of MWCNT was boiled at 100°C in 25mL of a 1:1 mixture of concentrated sulfuric and nitric acid under a condenser for1 hour. During this step, the reactive and highly strained CNT caps are removed, leaving open-ended tubes which facilitate ion transport during supercapacitor operation. The acid also cleans the side of the CNTs of dangling groups and bonds, leaving a clean surface which will not impede ion transport later. Finally, the acid shortens the tubes, which facilitates dispersion. Once all of the acid solution had been filtered, ethanol was filtered through the CNTs to wash them in order to stop the oxidation process. The MWCNT electrodes were then dried overnight in a vacuum oven at 80°C. This acid purification step was also done in order to introduce defects and surface functionalities onto the edge of the carbon nanotubes, which helps increase the rate of electron transfer and facilitates metal oxide deposition onto the MWCNTs during the ALD process. Four samples were investigated through the course of this study. An uncoated MWNT paper sample was compared with MWNT paper samples which had been coated with vanadium oxide through 100, 300, and 500 ALD cycles. The temperature of the furnace was maintained at 170-190°C. For electrochemical testing, the VOx-coated CNT electrodes were cut out to dimensions of 0.5x0.5cm. All supercapacitors were tested in beaker-cell configuration using gold current collectors (Sigma-Aldrich, USA) and separated by two layers of 25µm GorTex separator paper (GorTex, USA). We selected 8M LiCl solution (Sigma-Aldrich, USA) as an electrolyte because other common supercapacitor electrolytes (such as H2SO4) dissolved the VOx coating during electrochemical testing.
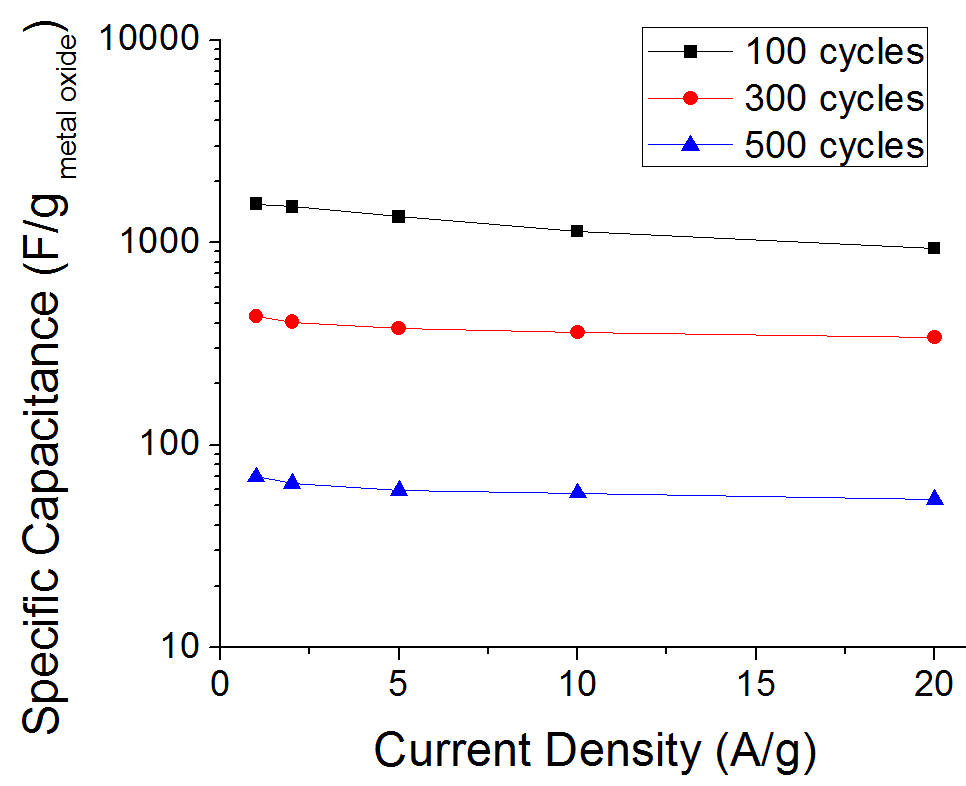
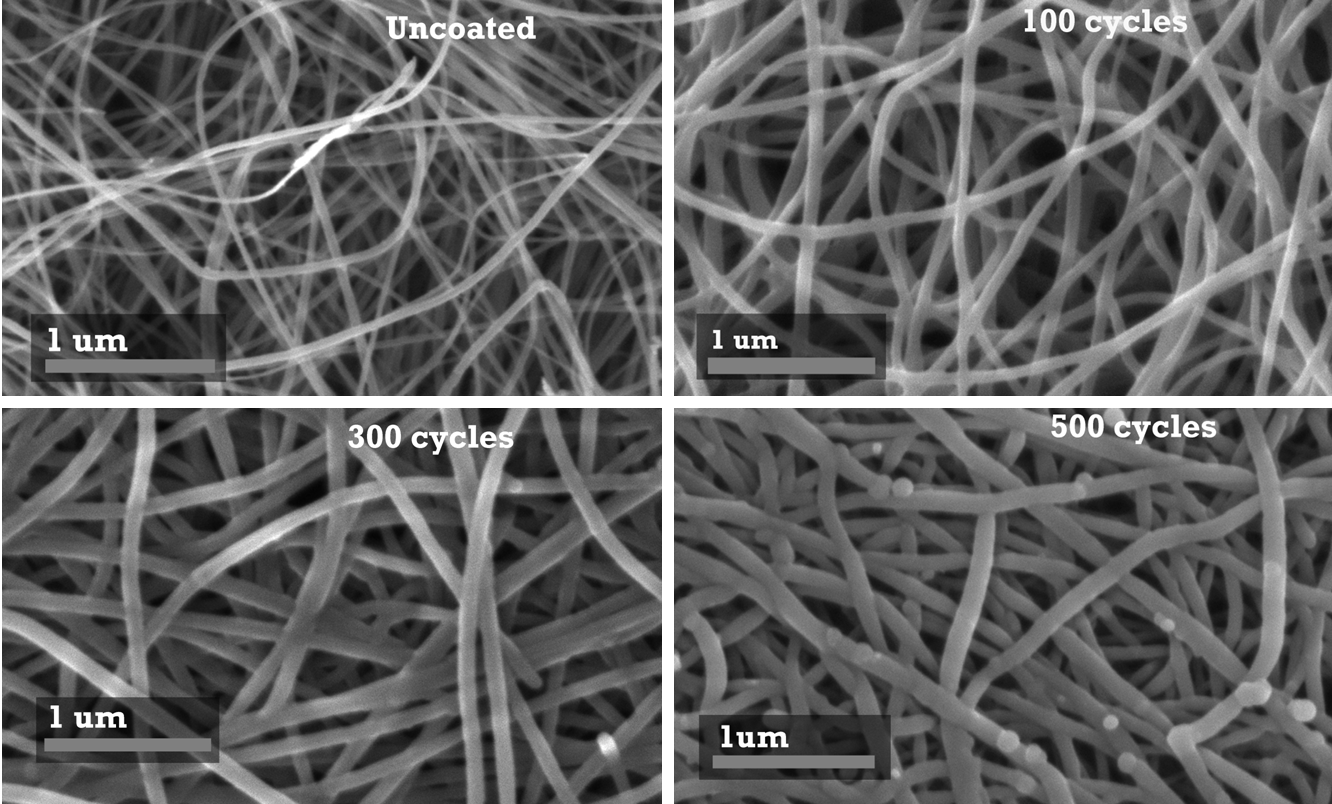
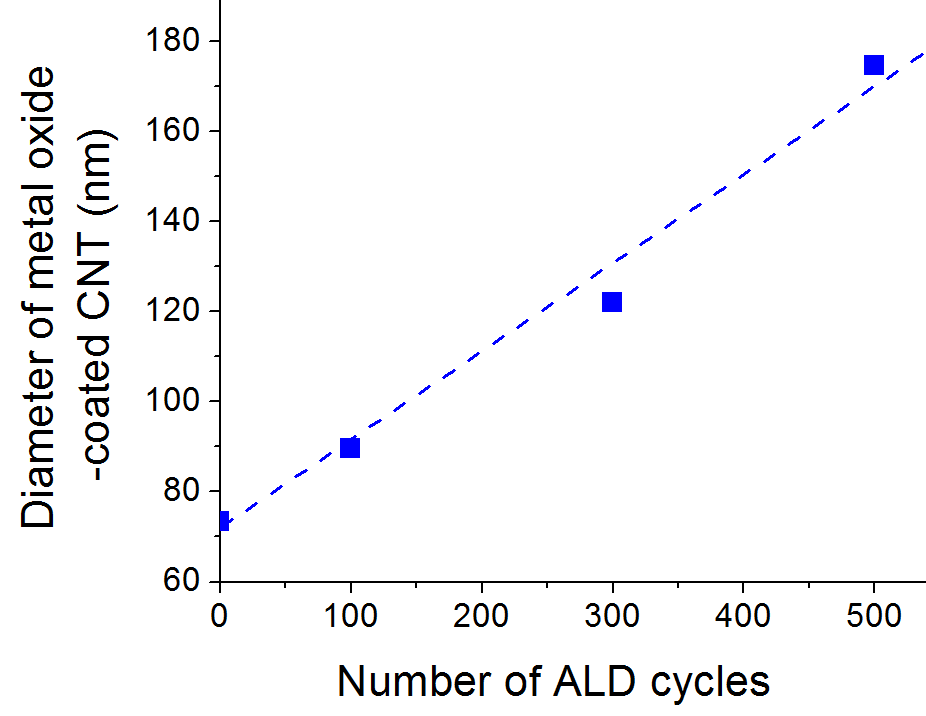
Optimization of the ALD deposition parameters and the chemical oxidation of the CNT surface allowed us to achieve uniform metal oxide coating on the CNT surface (Figure 1). Nearly linear change in the nanotube thickness was detected using SEM image analysis (Figure 2). Depending on the thickness of the deposited metal oxide layer, the specific capacitance of the produced material approached 1700 F/g (Figure 3), which is an order of magnitude higher than that of activated carbons conventionally used in commercial supercapacitor electrodes.
The conducted research was critical for the PI career as it revealed parameters needed to achieve uniform deposition of metal oxide films on the surface of carbon nanomaterials and the importance of the fine control over the structural properties of the produced carbon-metal oxide nanocomposites as well as the electrolyte-metal oxide interactions for achieving breakthroughs in the performance in energy storage devices.
The results of this work were covered in several international meetings:
1. G. Yushin, Nanocomposite Materials for High Energy Supercapacitors and Li-ion Batteries, at the 1st NSF-sponsored US-Taiwain Workshop for Materials and Systems Challenges in Electrical Energy Storage, Taipei, Taiwan (2011) - invited.
2. G. Yushin, Nanostructured Materials for Energy Storage Applications, at the 10X Advanced Battery R&D Conference, Santa Clara, CA, USA (2011) - invited.
3. G. Yushin, Nanocomposite Materials for Energy Storage Applications, at the 2nd UNIST International Conference, Ulsan, South Korea (2010) - invited.
4. G. Yushin, Nanocomposite Materials for Supercapacitors and Li-ion Batteries, North Carolina State University, Raleigh, NC, USA (2010) - invited.
5. B. Hertzberg, S. Boukhalfa, A. Magasinski, I. Kovalenko, P. Dixon, and G. Yushin, Carbon-Containing Nanocomposite Materials for Energy Storage, American Chemical Society, Boston, MA (2010) - invited.
6. S. Boukhalfa, A. Magasinski, B. Hertzberg, L. Wei, G. Yushin, Metal-Oxide / Carbon Nanocomposites for Use in Supercapacitors, 2010 World Conference on Carbon, Clemson, SC (2010).
7. S. Boukhalfa, A. Magasinski, G. Yushin, Metal-Oxide Coated Carbon Nanotube Electrodes for Use in Supercapacitors, 2011 International Conference from Nanoparticles and Nanomaterials to Nanodevices and Nanosystems, Crete, Greece (2011).
One of the graduate students involved in this project (Sofiane Boukhalfa) received NSF GSPC Award and a travel grant to participate in a 3rd International Conference from Nanoparticles and Nanomaterials to Nanodevices and Nanosystems (IC4N) and the Cretan Workshop on: Global Challenges and Opportunities for Nanotechnology, Crete, Greece.
| Figure 1. SEM micrographs of carbon nanotube electrodes before (top left) and after (top right and bottom) atomic layer deposition (ALD) of metal oxides: 100, 300 and 500 ALD cycles. A uniform coating is clearly visible in all samples. |
|
|