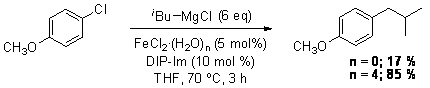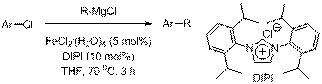www.acsprf.org
Reports: B148541-B1: N-Heterocyclic Carbenes in Iron-Catalyzed Cross-Coupling Reactions
Marc C. Perry, PhD , Point Loma Nazarene University
The three specific research objectives contained in the original proposal are listed below. I will discuss an overview of what was accomplished over the last three years and will provide more detail on what was accomplished since the last annual report.
1) Synthesize iron(II) imidazol-2-ylidene and imidazolin-2-ylidene complexes and test their reactivity in the coupling of secondary Grignards to aryl chlorides, and study the same reaction using iron(II) imidazol-2-ylidene and imidazolin-2-ylidene complexes formed in situ by the deprotonation of the corresponding azolium salt with a Grignard as base.
2) Test the feasibility of using these iron(II) complexes in the Sonogashira reaction using aryl chlorides
3) Test the feasibility of using these iron(II) complexes in the a-arylation of 1,3-dicarbonyls using aryl chlorides.
Over the past three years, attempts to realize specific research objectives 2 and 3 have failed, and cross-couplings of terminal alkynes and 1,3-dicarbonyls with aryl chlorides have not been realized. Regardless of attempts to couple these substrates under a variety of reaction conditions, the starting aryl chloride was always recovered.
A significant amount of time was also spent trying to isolate discrete iron(II) imidazole-2-ylidene complexes by reacting imidazole-2-ylidenes with FeCl2. Regardless of the specific reaction conditions used, highly insoluble material was isolated that contained both iron and the imidazol-2-ylidene components, but was likely oligomeric in nature. The cross-couplings between aryl chorides and alkyl Grignard reagents were, therefore, carried out under conditions where the imidazole-2-ylideneiron complexes were formed in situ.
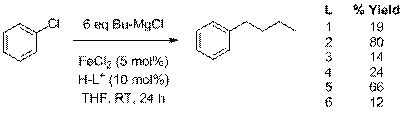
In order to test the viability of using N-heterocyclic carbenes as ligands in the iron-catalyzed cross-coupling of alkyl Grignard reagents and aryl chlorides, butylmagnesium chloride was added to THF solutions containing chlorobenzene, anhydrous FeCl2 (5 mol%), and imidazolium chlorides or imidazolinium tetrafluoroborate (10 mol%). The imidazolium and imidazolinium salts were converted into the corresponding imidazol-2-ylidines and imidazolin-2-ylidines in situ upon reaction with the strongly basic Grignard reagents. The different N-heterocyclic carbenes that were generated in situ and used in this study are shown in Scheme 1, and the results obtained are shown in Scheme 2.
Scheme 1. NHC ligands used
Scheme 2. Ligand screening
All of the ligands resulted in some noticeable coupling, but ligand 2, bearing the bulky diisopropylphenyl groups, gave the best overall result. We were delighted with these results as non-activated aryl chlorides like chlorobenzene have yet to be effective in iron-catalyzed cross-coupling reactions of alkyl Grignard reagents. After these promising initial resluts, we investigated the use of ligand 2 in the iron-catalyzed cross coupling of three non-activated aryl chlorides with a number of primary and secondary alkyl Grignard reagents. The reactions were run at 70 oC for three hours in THF. The aryl chlorides used were chlorobenzene, 4-chlorotoluene, and 4-chloroanisole, and the Grignard reagents used were propyl-, butyl-, isobutyl-, cyclohexyl-, isopropyl-, and sec-butylmagnesium chloride. The primary alkyl Grignard reagents coupled in excellent yields with all three aryl chlorides with yields in the 80's and high 90's. A complete table of the data has been included in a previous annual report.
This work was submitted to Tetrahedron Letters, and the single reviewer felt it was not publishable without obtaining some isolated yields. Unfortunately, repeated attempts at scaling up the reaction failed to give equivalent yields as the runs described earlier. While trying to understand this phenomenon, a paper was published describing the use of NiCl2 catalysts for Kumada couplings involving tertiary Grignard reagents. The authors realized that inconsistencies in their reactions were due to differences in the amount of trace water. They found out that 1.5 equivalents of water relative to the Ni-catalyst provided much better yields than if truly anhydrous catalyst was used. In order to investigate if the same could be true of our system, we compared the results of the cross-coupling of p-chloroanisole with isobutylmagnesium chloride using anhydrous FeCl2 and FeCl2·(H2O)4 as catalyst. The results are shown below.
Interestingly, FeCl2·(H2O)4 was found to be a superior catalyst in the cross-couplings carried out in our lab. We, therefore, went back and ran the cross-couplings reported previousle using the FeCl2·(H2O)4 catalyst instead. The results are shown in Table 1. As with the anhydrous catalyst, primary alkyl Grignard reagents coupled in excellent yields while the secondary cyclohexylmagnesium chloride coupled in poor to moderate yields.
Table 1. Cross-Coupling using FeCl2·(H2O)4
Entry | R | Ar | Methoda | % Yieldb |
1 | isobutyl | Ph | A | 98 |
2 | isobutyl | p-CH3-Ph | A | 96 |
3 | isobutyl | p-CH3O-Ph | A | 98 |
4 | butyl | Ph | A | 96 |
5 | butyl | p-CH3-Ph | A | 89 |
6 | butyl | p-CH3O-Ph | A | 83 |
7 | propyl | Ph | A | 98 |
8 | propyl | p-CH3-Ph | A | 84 |
9 | propyl | p-CH3-Ph | B | 94 |
10 | propyl | p-CH3O-Ph | A | 79 |
11 | propyl | p-CH3O-Ph | B | 90 |
12 | cyclohexyl | Ph | A | 58 |
13 | cyclohexyl | Ph | B | 65 |
14 | cyclohexyl | p-CH3-Ph | A | 47 |
15 | cyclohexyl | p-CH3-Ph | B | 50 |
16 | cyclohexyl | p-CH3O-Ph | A | 45 |
17 | cyclohexyl | p-CH3O-Ph | B | 43 |
a) Method A involved using 3 eq of the alkyl Grignard reagent added in one portion at the beginning of the reaction; Method B involved using 4 eq of the alkyl Grignard added in two equal portions over the course of the reaction. b) Yields determined by GC using naphthalene as an internal standard.
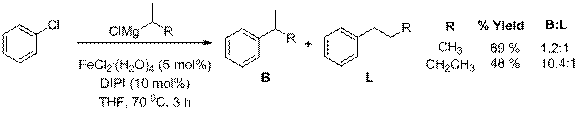
The cross-couplings of isopropyl- and sec-butylmagnesium chloride were then attempted using chlorobenzene as shown in Scheme 3. In both cases the desired cross-coupling product was accompanied by an n-alkyl isomer resulting from reversible β-hydrogen elimination competing with reductive elimination in the catalytic cycle. This side reaction is well known for cross-coupling reactions involving secondary organometallics, and is evidence that the mechanism is ionic in nature.
The salary for three undergraduate students and their supplies were completely funded by the ACS-PRF monies. These students were able to participate in a summer research program in which they worked full time for 10 weeks under my supervision.