www.acsprf.org
Reports: ND149817-ND1: Photocatalysis with Visible Light
Tehshik P. Yoon , University of Wisconsin (Madison)
RESEARCH PROGRESS
This research program investigates synthetically useful photochemical reactions promoted by visible light. We have made significant progress towards our goals:
1. We have demonstrated that ruthenium polypyridyl complexes are highly effective catalysts for visible light photocatalysis.
2. We have developed a suite of efficient, synthetically useful cycloadditions, including [2+2], [3+2], and [4+2] processes, that involve a variety of electron-rich and electron-deficient olefinic substrates. This diverse reactivity is enabled by the ability of photoexcited Ru(bpy)32+ complexes to participate in both reductive and oxidative catalytic cycles.
3. We have probed the mechanism of the reductive catalytic cycle and have developed a good understanding of the role of the various reaction components. As a result, we have developed the capacity to rationally tune reaction parameters to effect novel chemical transformations.
4. We have demonstrated the applicability of our new methods to synthesis by constructing the natural product heitziamide A, a relatively simple biologically active compound that is only synthetically accessible using visible light photocatalysis.
Photoreductive processes
We have shown that Ru(bpy)32+ complexes can catalyze the reductive of electron-deficient enones to the corresponding radical anions, and that these photogenerated radical anions undergo a variety of reactions, including [2+2], [3+2], and [4+2] cycloadditions and reductive coupling reactions:
Elucidation of the mechanisms of the reactions developed in our laboratory is a central theme of the research in my group. The development and optimization of these processes in my laboratory have been guided by our understanding of the mechanism of catalysis and by the ability to tune the identity of Lewis acidic and Lewis basic additives.
Photooxidative processes
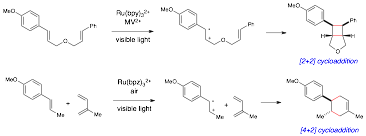
An attractive feature of our strategy for photocatalysis is the electrochemical versatility of Ru(bpy)32+ and related chromophores. We have utilized the ability of these photocatalysts to perform oxidative processes to develop complementary methods that can engage electron-rich olefins in [2+2] and [4+2] cycloadditions via the corresponding radical cations:
The success of these processes was dependent on two key observations. (1) The oxidation potential of the photocatalyst can be increased by the appropriate choice of oxidative co-oxidant, which activates the ruthenium complex towards oxidative processes. (2) Due to the interest in ruthenium polypyridyl catalysts as photosensitizers in solar energy applications, the effect of ligand modifications on the electrochemical and photophysical properties of these complexes is well understood, and many Ru(bpy)32+ derivatives are known with a range of oxidation potentials.
Synthetic applications
This project is relevant to the mission of NIGMS because photochemical processes can produce molecular structures that are not accessible using normal thermal methodology. To highlight this fact, we have completed a synthesis of the natural product heitziamide A. The regiochemistry of the key Diels–Alder cycloaddition can only be produced by a photosensitized radical cation process and not by the corresponding thermal or Lewis acid catalyzed cycloaddition.
SIGNIFICANCE. We are developing powerful synthetic methods that are designed to make photochemical reactions accessible to the synthetic organic chemistry community and therefore enable the discovery of new bioactive compounds in areas of otherwise unexplored chemical diversity space.
PLANS. Our major efforts for the upcoming year of NIH support are summarized below.
1. We will continue our open-ended exploration of synthetically useful reactions involving photogenerated radical ion intermediates.
2. In order to broaden the synthetic utility of this strategy, we will develop "redox auxiliaries" to tune the electrochemical properties of alkene substrates such that they are amenable to the one-electron redox processes involved in our methods.
3. We will develop methods to control the absolute stereochemistry of the photoinduced processes developed in our labs.
4. We will continue to apply these reactions to the synthesis of bioactive natural products and new biologically active small molecules whose synthesis is uniquely enabled by photocatalytic methods.