www.acsprf.org
Reports: DNI650223-DNI6: Vibrational Spectroscopy of Transient Combustion Intermediates Trapped in Helium Nanodroplets
Gary E. Douberly, PhD , University of Georgia
During this first year of funding, we have made significant progress towards our research goal of implementing helium nanodroplet isolation to trap and stabilize intermediates of prototype reactions that are central to combustion chemistry. Intermediates that result from the barrierless reactions between hydrocarbon radicals or between a hydrocarbon radical and molecular oxygen are being characterized with infrared laser spectroscopy. The vibrational frequencies, dipole moments, and vibrational transition moment angles obtained from these studies add new chemical insight into the detailed mechanisms of reactions that are relevant to low-temperature hydrocarbon oxidation and soot formation.
We have completed the construction of a continuous, effusive pyrolysis source for doping helium droplets with hydrocarbon radicals. Radicals are generated by pyrolysis as precursor molecules collide with the walls of a heated quartz tube. This effusive radical beam crosses the path of a helium droplet beam, and the concentration of radicals in the pick-up zone is controlled such that each droplet picks up only one radical. Multiple pick-up zones are employed to introduce different radicals to the same droplets for reaction studies.
Figures 1 and 2 demonstrate the use of the pyrolysis source for doping droplets with the methyl radical (CH3). The pressure in the source is kept low such that each droplet picks up only one precursor molecule on average. Peaks in the mass spectrum due to the ionization of the solvated precursor are evident at (m/z=43, 57, and 73). Upon heating the pyrolysis source to 530 K, the peaks due to the precursor are reduced and the m/z=15 peak grows in intensity, signaling the pyrolysis of DTBP and the production of methyl radicals.
Figure 1: a) Mass spectrum of the helium droplet beam with the pyrolysis source off. b) Mass spectrum of the droplet beam with the DTBP precursor being bled through the quartz tube such that each droplet picks-up one DTBP molecule on average. c) Mass spectrum while the precursor is being pyrolyzed at 530 K, showing the appearance of m/z=15 (CH3+).The spectrum of the methyl radical (Figure 2) is obtained by overlapping the droplet beam with the output from an infrared laser system. Vibrational excitation of the solvated methyl radical followed by vibrational relaxation results in a droplet size reduction (~630 He atoms for this CH stretch excitation at 3150 cm-1). This size reduction decreases the electron impact probability of the methyl doped droplets as they enter into the mass spectrometer. The IR spectrum shown in Figure 2 (middle) corresponds to the depletion of the m/z=15 ion signal (CH3+) as the laser is tuned through the ro-vibrational resonances of the methyl radical. Monitoring depletion signals in specific mass channels provides a selective method that eliminates signals from droplets containing un-pyrolyzed precursor molecules or other products of pyrolysis. A simulation of the methyl radical spectrum (Figure 2, bottom) based on an oblate top confirms the assignment and demonstrates the weak effects of the helium on the spectrum.
Figure 2: CH3 (e) degenerate asymmetric CH stretch spectrum measured in the m/z=15 mass channel. The red curve corresponds to a simulated spectrum assuming an oblate symmetric top. The assignments of each ro-vibrational transition are shown in the notation DKDJK′′ (J′′). The top frame corresponds to the difference mass spectrum with the laser fixed to the peak of the RR0(0) transition at 3174.22 cm-1. The difference spectrum clearly shows that the majority of the beam depletion occurs in the m/z=15 mass channel, which is to be expected for the methyl radical, based on its known electron impact mass spectrum.Figure 3 shows our recent measurement of the dipole moment of the ethyl radical (C2H5). Ethyl radicals can be generated by the pyrolysis of multiple precursors, including di-tertamyl peroxide, n-propyl nitrite, and ethyl iodide. The top zero-field spectrum in Figure 3 corresponds to the ethyl radical CH2 symmetric stretch ro-vibrational spectrum. The simulation of the Stark spectrum (Figure 3; bottom red curve) is obtained assuming a dipole moment of 0.28(2) Debye. This measurement represents the first determination of the ethyl radical's permanent electric dipole moment.
Figure 3: Ro-vibrational Zero-field (top) and Stark (bottom) spectra of the CH2 symmetric stretch of the ethyl radical. The red traces correspond to simulations. The Stark spectrum is simulated with 0.28(2) Debye dipole moments for both the ground and excited vibrational states.We have also made progress on studies in which a reaction occurs between two radicals picked-up by a single helium droplet. Of particular interest is the reaction between resonantly stabilized free radicals (RSFRs), such as the propargyl (C3H3) and allyl (C3H5) radicals, with O2. Unlike alkyl + O2 reactions, quantum chemistry predicts barriers of ~1-4 kcal/mol in the entrance channels of the C3H3 + O2 and C3H5 + O2 reactions. Upon forming the C-O bond, the resonance stabilization energy in these hydrocarbon radicals (~8-9 kcal/mol) must be broken, hence the presence of the small entrance channel barrier. It is therefore interesting to consider the condensation of C3H3 and O2 within a helium droplet. From previous helium droplet work, we expect to observe the metastable entrance channel C3H3-O2 van-der-Waals complex.
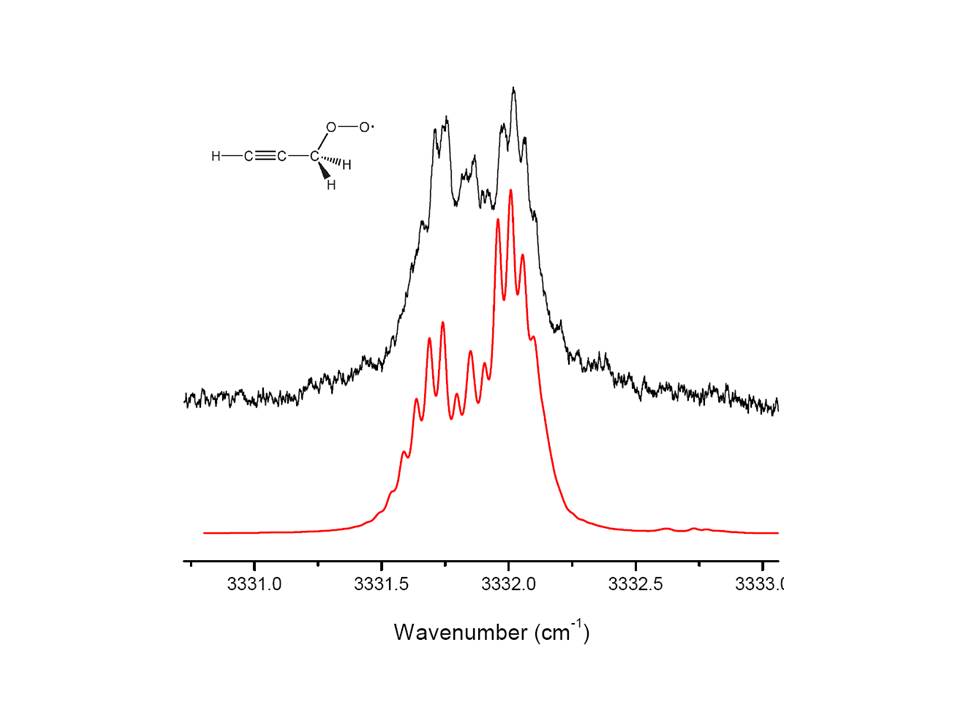
However, upon introducing single O2 molecules to droplets containing single propargyl radicals, we find no evidence for the formation of a weakly bound C3H3-O2 van-der-Waals complex. Instead, we find a band in the spectrum that can be assigned to the propargyl peroxy radical (Figure 4), an intermediate on the C3H3-O2 potential surface, which is about 20 kcal/mol downhill from the entrance channel. This result suggests that the barrier height may be overestimated by the single reference ab initio calculations for this system. Indeed, it has been shown that the transition state region of the potential surface exhibits strong multi-reference character. We are continuing this study and plan to conduct multi-reference ab initio calculations of the entrance channel valley of the C3H3-O2 potential surface.
Figure 4: Ro-vibrational spectrum of the acetylenic CH stretch of the propargyl peroxy radical. The red simulation is based on the computed rotational constants for the trans-acetylenic isomer.