www.acsprf.org
Reports: DNI749881-DNI7: Understanding the Diffusion of Polymers Confined between Interfaces
Christopher J. Ellison, PhD , University of Texas (Austin)
The dynamic behavior of polymers near interfaces is critical to performance in applications in which the diffusion of polymer chains parallel to nanoconfining interfaces can play an important role. For example, in nanocomposites the diffusion of polymers along sheet-like filler particles is different from bulk diffusion which can affect polymer crystallization behavior and exfoliation; both are key to achieving desired physical properties, such as high modulus. Unfortunately, it is not well understood how nanoconfinement by interfaces generally impacts all dynamic molecular processes. The focus of this research is on the longest dynamic process of self-diffusion.
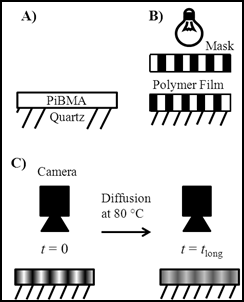
To study whole polymer chain mobility, a new fluorescence recovery after photobleaching (FRAP) visualization approach was developed and used to monitor diffusion of polymers confined in thin films. FRAP is a well-established technique for tracking species diffusion in quantitative biology, but has not received significant attention in materials science [1-3]. Figure 1 shows a cartoon schematic of a typical FRAP experiment where diffusion of a polymer with a covalently attached fluorescent probe (i.e., labeled polymer) is visualized.
Figure 1: Schematic of the FRAP experiment. A) Spin coating and annealing of a labeled polymer film. B) Photobleaching with a high intensity light through a mask to initially create a periodic array of fluorescent and non-fluorescent regions. C) Redistribution of fluorophores from t=0 to t=tlong as diffusion occurs with associated relaxation in the pattern. Light areas indicate regions of high fluorescent intensity and dark regions indicate regions of low fluorescent intensity.
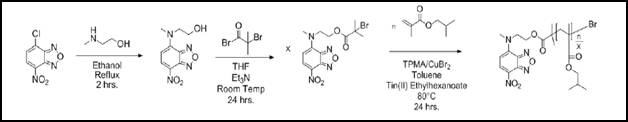
For this study, poly(isobutyl methacrylate) (PiBMA) was chosen as a model system. The polymer was synthesized to have a single fluorophore (7-nitrobenzofurazan or NBD) covalently incorporated into the polymer (PiBMA-NBD). This fluorophore containing molecule was used to initiate polymerization via ARGET ATRP. This approach yielded polymer with a narrow molecular weight distribution (Mn = 8,600 g/mol and PDI = 1.1) in which every chain contained one fluorescence end group (PiBMA-NBD). The synthetic scheme used to produce this polymer is shown in Figure 2.
Figure 2: Synthesis of NBD labeled PiBMA by ARGET ATRP
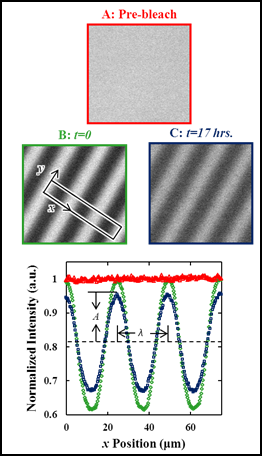
Figure 3 shows a series of images prebleach, postbleach, and after 17 hours of diffusion. The period of the photobleached pattern is 25 μm. As shown in Figure 3, the diffusion of fluorophores from fluorophore-rich regions (protected by the mask during photo bleaching) to fluorophore-poor regions (exposed by the mask during photo bleaching) is observed.
Figure 3: Fluorescence micrographs A) before photobleaching (r) B) at the start of the diffusion experiment after patterned photobleaching and annealing (¯) and C) after significant recovery (£) of a 127 nm film of PiBMA-NBD. Lower plot be shows the normalized intensity as function of position (x) from a 300 pixel wide (in y) line integral perpendicular to the bleached pattern over several periods.
The intensity profile of a photobleached array of lines and spaces can be well described by a sinusoid whose time evolution allows for a direct calculation of D. Using this technique we have been able to measure the bulk diffusion coefficient for thick films of PiBMA and poly(methyl methacrylate), and films of PiBMA as thin as 30 nm.
In addition to funding research for developing this new visualization approach for tracking diffusion, The American Chemical Society – Petroleum Research Fund has provided catalytic funding for a National Science Foundation CAREER grant which will fund the continued research for the next five years. Additionally, the results from this study have been presented in a variety of national conferences including a poster session at the American Chemical Society National Meeting in spring of 2010, an oral presentation given by the graduate student at the meeting of the American Physical Society in spring 2011, and finally by the PI in an invited talk at the American Chemical Society Fall 2011 National Meeting. All of these meetings created opportunities for forging collaboration outside the university. Finally, a manuscript entitled “Self-Diffusion of Nanoconfined Poly(isobutyl methacrylate) Parallel to Interfaces” is nearing completion for submission to the journal Macromolecules.
This grant supported one graduate student who is working on this project as the primary focus of his doctoral research and provided an essential opportunity to learn how to begin a research project that will benefit him in his future career. Using the travel allowance, he was able to attend all of the conferences to present this research. This allowed him not only to meet many of the leading names in the field, but also to get a better and broader picture of the state of polymer research.
References
1. Granick, S.; Bae, S. C., Open questions about polymer interfacial diffusion. J. Polym. Sci. Pt. B-Polym. Phys. 2006, 24.
2. Axelrod, D.; Koppel, D. E.; Schlessinger, J.; Elson, E.; Webb, W. W., Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976, 9.
3. Sprague, B. L.; McNally, J. G., FRAP analysis of binding: proper and fitting. Trends Cell Biol. 2005, 2.