www.acsprf.org
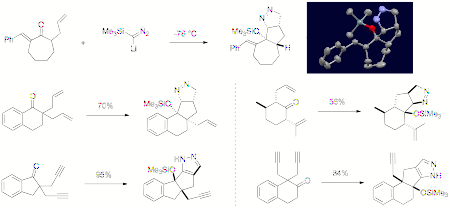
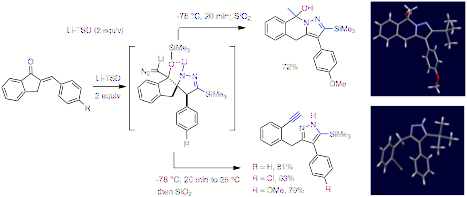
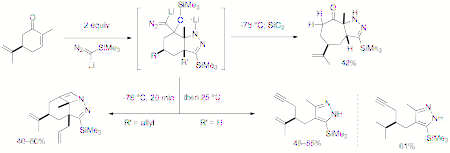
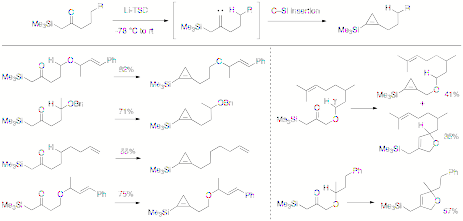
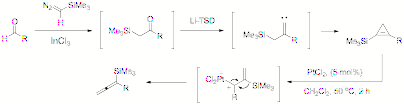
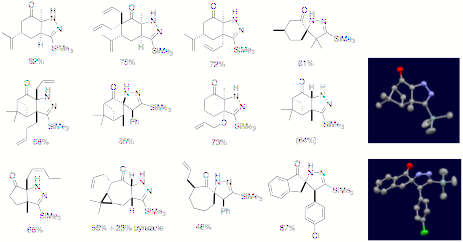
Reports: ND149836-ND1: New Cycloaddition Reactions of Anionically Activated Dipoles
Daesung Lee, PhD , University of Illinois (Chicago)
The supported of the ACS Petroleum ResearchFund allowed the PI to broaden his research scope and theme into a new areacompletely unrelated to what he has been engaged in. This expansion of researchscope was not possible without the support. This new research developedand executed under the financial support not only allow the PI to explore newchemistry reshaping the research direction, but alsogives a profound impact on the career of the graduate students engaged in theresearch. Especially, these students did more exploratory research in anunknown territory rather than just expanding and embellishing thewell-established research of the PI such that they should have a greatopportunity to become a more intellectually developed and independentresearcher. ![]()