www.acsprf.org
Reports: DNI350395-DNI3: Strategies for the Reduction of Carbon Dioxide to Methanol
Connie Lu, PhD , University of Minnesota
Goal 1: Understanding the Chemical Foundations for Carbon Dioxide Reduction to Methanol Using Model Systems
1.1 A Nickel Diphosphine System Akin to Aresta's Complex
We previously identified Aresta's nickel system (PCy3)2Ni as a potential framework for making and studying metal-C1 complexes. Several metal-C1 species that mimic hypothetical intermediates in a CO2-to-methanol reduction scheme were targeted. Initial efforts to isolate the CO2 adduct, (PCy3)2Ni(h2-CO2) were unsuccessful, producing an intractable mixture. One possible problem is the dissociation of the phosphine ligand from the nickel center, leading to scrambling.
Recently, we developed a wide bite-angle diphosphine ligand, 4,6-bis(3-diisopropylphosphinophenyl)dibenzofuran (abbreviated as iPrDPDBFphos, shown in Scheme 1). Due to the chelate effect, a diphosphine ligand would hinder dissociation from the metal center. A unique advantage of iPrDPDBFphos is its conformational flexibility: the diphosphine can adopt a wide range of bite angles within coordination complexes, from 105 to 180 degrees. In the CO2-to-methanol catalytic cycle, the transition metal catalyst undergoes one and two-electron redox reactions, and the resultant changes in the metal oxidation state is typically accompanied by changes in the coordination geometry. Hence, a ligand that can accommodate different geometries and that resists de-chelation, is useful to any catalytic application.
1.1.1 Synthesis of ( iPrDPDBFphos)Nickel Complexes and their Characterization
The metallation of the iPrDPDBFphos ligand with NiCl2 produced (trans-iPrDPDBFphos)NiCl2. Using a nickel(0) precursor Ni(COD)2 (where COD = 1,5-cyclooctadiene), metallation reactions lead to a mixture of products. The reaction will proceed cleanly if a stoichiometric equivalent of benzophenone is added, generating (cis-iPrDPDBFphos)Ni(h2-OCPh2). The Ni-benzophenone adduct is an analogue of an intermediate in the postulated CO2-to-methanol cycle: LNi(h2-OCH2). Direct synthesis of LNi(h2-OCH2) from Ni(COD)2 and paraformaldehyde resulted in oxidation of formaldehyde to CO, and (trans-iPrDPDBFphos)Ni(CO)2 was isolated in good yield. Though not the desired product of the reaction, a CO adduct is also an intermediate in the CO2-to-methanol cycle. Other nickel complexes were prepared, e.g. (iPrDPDBFphos)NiCl and (trans-iPrDPDBFphos)Ni(CH2Cl)Cl, which are potentially useful precursors for other C1-intermediates.
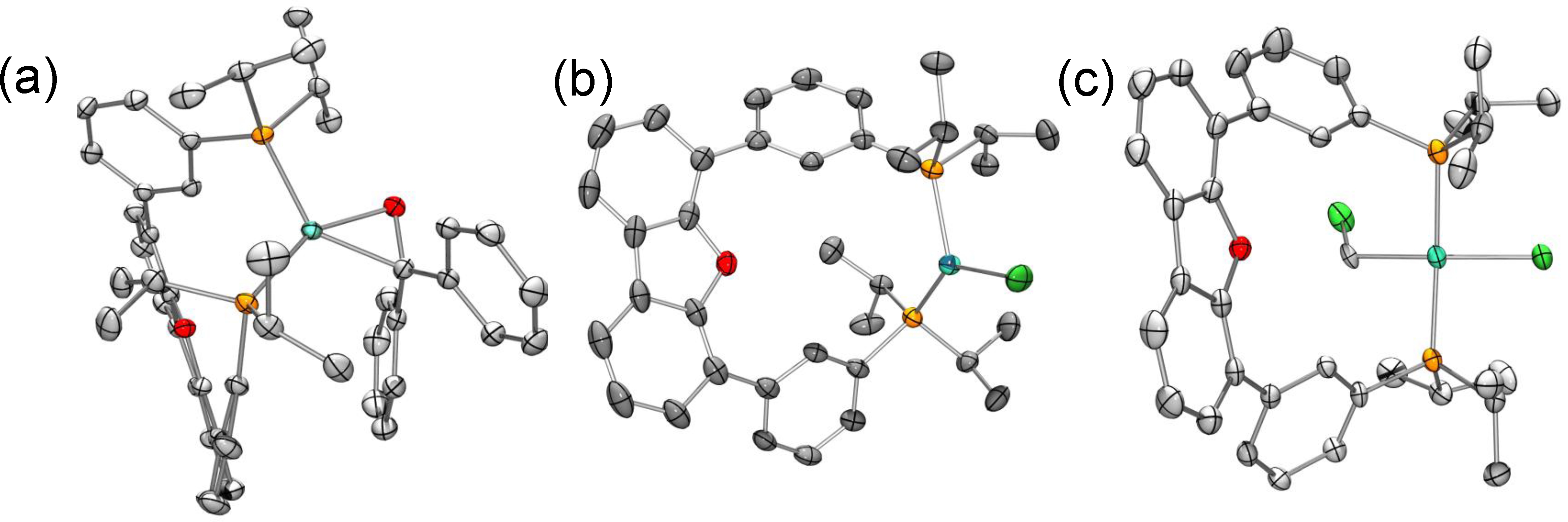
Figure 1. Solid-state structures of (a) LNiII(h2-OCH2), LNiICl, and LNi(CH2Cl)Cl.
Goal 2: Develop New Homogeneous Systems for CO2 Reduction Chemistry
2.1 Development of Metal-Cage Systems.
2.1.1 Computational Studies of Metal-Cage Systems.
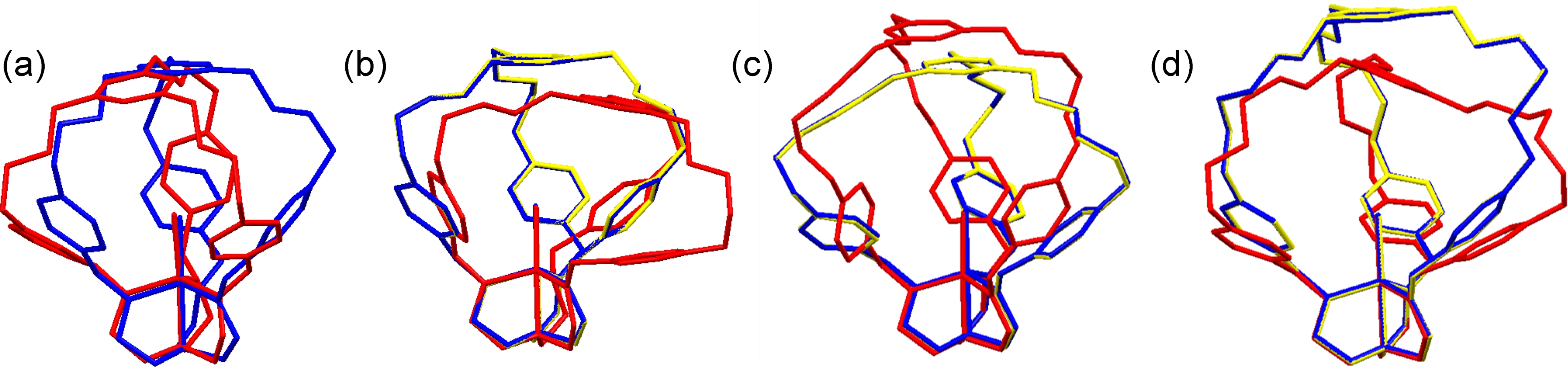
A set of density functional theory calculations were completed to visualize the metal-cage targets containing varying ligand arm lengths. The ligand arms were systematically varied from four to seven methylene units for a hypothetical titanium chloride compound. The objective of this study was to measure cavity heights. The cavities must be sufficiently large to accommodate carbon dioxide. The calculations were run with and without symmetry constraints to sample the range of cavity heights available. Pictures of various optimized conformations are shown in Figure 2.
Figure 2. Geometry Optimized DFT Models of Titanium Chloride Cage Complexes with Varying Ligand Arm Lengths (4 - 7 methylene linkers, (a) - (d) respectively).
As the methylene linkers are increased, the floppiness of the metal-cage systems are apparent. Furthermore, the energy difference between the conformations increases. For example, in the 4-linker case (a), the energy difference between the two conformation is 0.2 kcal/mol. The energy difference jumps to 6 kcal/mol in (b) and to 9 kcal/mol in (d). At room temperature, fluxionality of the metal-cage complexes is expected to be facile. This may pose a potential problem in obtaining single crystals for X-ray structural studies. The cavity height is 7.55 Å in (a), from the Ti center to the centroid of the aryl cap. As the distance was deemed sufficiently large for CO2, the synthetic efforts focused on preparing the four methylene linker cage ligand.
2.1.2 Synthesis of Cage Ligands.
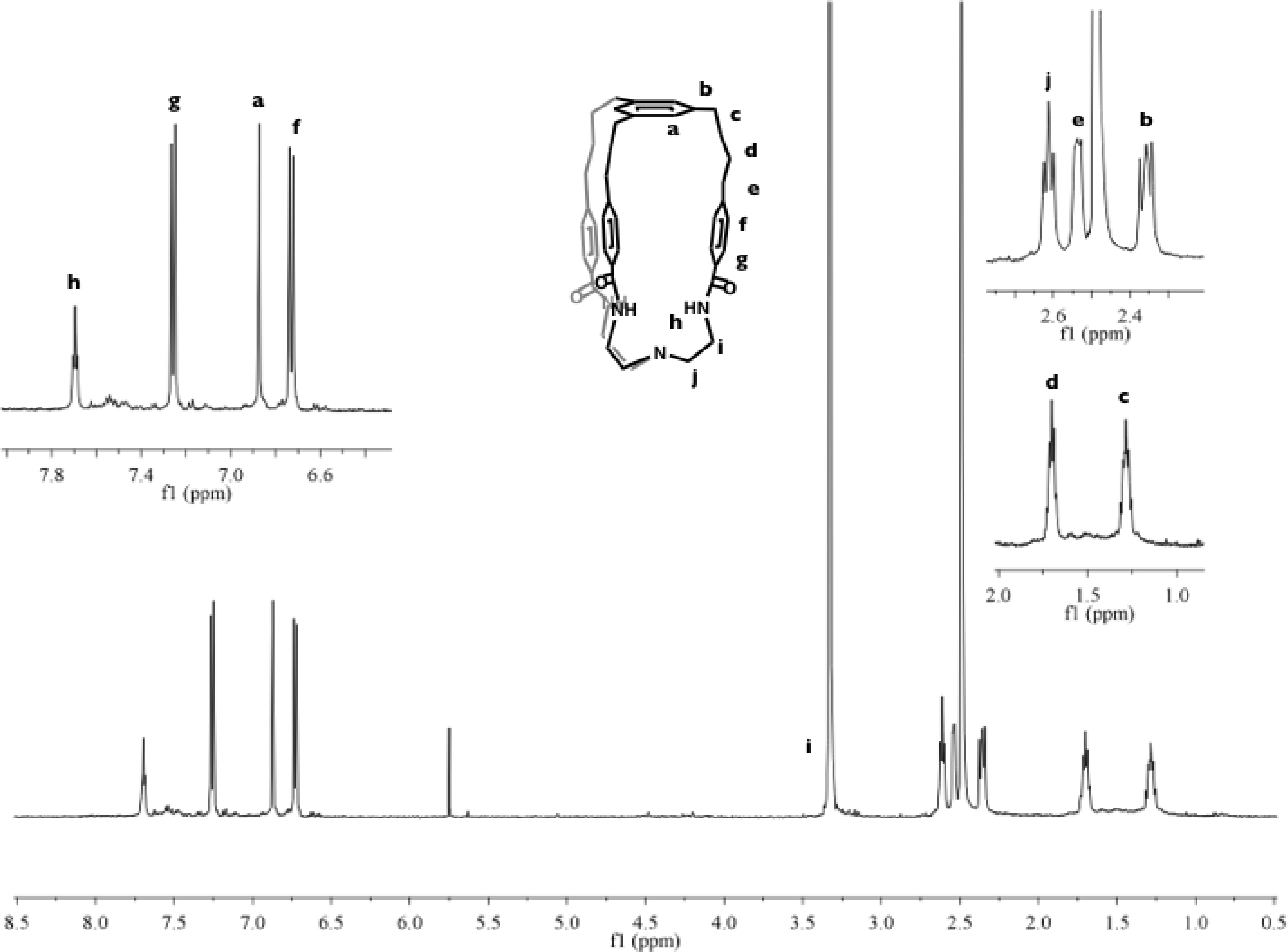
The bulk of our work this past year focused on preparing cage ligands. These ligands are conceptually new and synthetically challenging to prepare. The key synthetic step is the controlled coupling of the all three arms from the two parts of the cage ligand (simply called the cap and bottom) to create a discrete molecule rather than oligomers and/or polymers. Our initial efforts focused on using palladium catalyst for joining the two parts via triple carbon-carbon bond coupling formation in a one-pot Suzuki-Miyaura catalytic reaction. Both parts were independently prepared (2 to 3 steps each) to allow for a convergent synthesis (Scheme 2 (a)). However, isolated yields of the target cage ligand were low (~10 %) and extensive purifications by column chromatography was required. Because of the impractical nature of the coupling step, an alternative strategy (b) was pursued. In the top-down approach (b), the cap is completely built up until it is ready to combine with commercially available tris(2-aminoethyl)amine, i.e. tren, to form the targeted cage ligand. A special reagent, 2,2'-dipyridyldisulfide, was used to form the three lactam arms. The reaction appears nearly quantitative by crude proton NMR spectroscopy, and the target cage ligand has been isolated on a 1 g scale in moderate yields (65%). The cage ligand is soluble in DMSO and DMA, and its proton NMR spectrum is shown in Figure 3.
Figure 3. 500 MHz Proton NMR Spectrum of Cage Ligand in d6-DMSO.
2.1.2 Synthesis of Metal-Cage Systems.
For exploring metal-cage systems, zinc is a logical choice for the metal because it is inexpensive, air stable and diamagnetic. A Zn complex of the cage ligand was synthesized, and a crystal structure (TOC graphic) revealed a cavity height of 9.58 Å! Future work during the grant period will focus on inserting redox-active metals into the cage framework and studying small-molecule binding with these unique coordination complexes.
Impact:
Receiving this grant at the end of my first year gave me considerable freedom to undertake risky research ideas. My graduate students who worked on the cage ligand were excited to be funded by the American Chemical Society. Funds from this grant have also paid for summer work by undergraduate researchers. Not only was research a great learning experience for the two undergraduates, but they were instrumental in providing the extra manpower needed for this project.