www.acsprf.org
Reports: AC747700-AC7: ROMP Healing Agent Development for Self-Healing Materials
Michael R. Kessler , Iowa State University
In the past decade, polymers that can repair themselves with autonomy have been extensively studied in academia and received commercial interest [[1],[2]]. Perhaps the most successful self-healing mechanism to date incorporates liquid healing agent-filled microcapsules and catalyst particles into a polymer matrix. Upon fracture, microcapsules rupture, followed by flow of the healing agent into the crack volume. When the healing agent contacts the catalyst particles it polymerizes and adheres the crack faces together [[3]]. The most well-studied healing agent/initiator combination used in self-healing systems is dicyclopentadiene (DCPD) and Grubbs' catalyst, the former of which undergoes ring-opening metathesis polymerization (ROMP) in the presence of the latter. Despite the fact that these materials are on the verge of commercial implementation, there are still a number of questions as to why and how healing agents with different properties affect the healing mechanism. It is believed that one of the reasons why some of these questions have gone unanswered is due to analytical limitations; current analytical norms to study self-healing materials lack the versatility and practicality to adequately construct experiments designed to probe the effect of healing agent properties on self-healing. To this end, building off of the PI's previous work [[4], [5]], we have developed a modified rheokinetic technique that is designed to mimic self-healing.
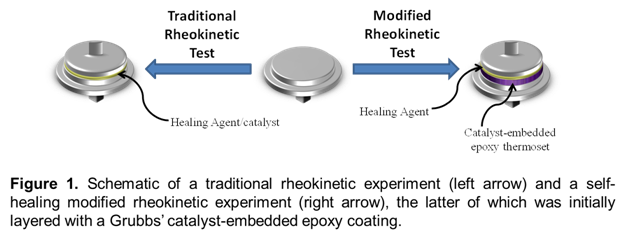
In a standard rheokinetic curing test, monomer is premixed with catalyst outside of the rheometer instrument, and the resulting solution is injected between rheometer parallel plates (Figure 1, left). In our modified technique, the bottom parallel plate is coated with a layer of epoxy polymer containing embedded Grubbs' catalyst, and the coating is polished to reveal catalyst. Monomer (without pre-dissolved catalyst) is then injected between modified bottom parallel plates (Figure 1, right), in which the monomer must dissolve embedded catalyst from the plate surface as it polymerizes. This technique mimics the healing mechanism while allowing for the rheological properties of the curing healing agent to be monitored under a number of different external stimuli. The effect of certain external stimuli believed to be important factors in the self-healing mechanism are discussed below.
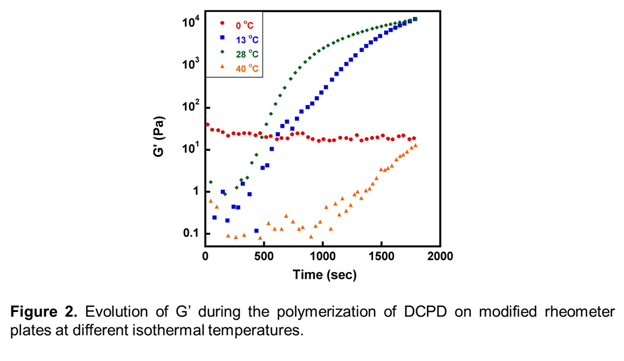
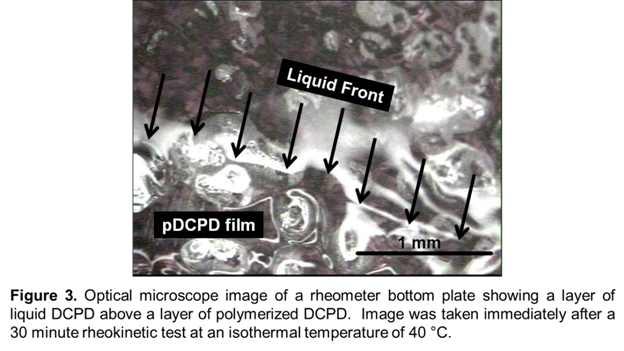
Given that self-healing polymers are envisioned for outdoor applications, in which temperatures fluctuate based on the time of day, season, and global location, it is of interest to obtain a detailed understanding of how self-healing changes with temperature. Figure 2 shows the change in G' of DCPD at a different isothermal temperatures, chosen to mimic what may be experienced in outdoor environments. At 0 °C, there is no observable growth of G'. This is likely because 0 °C is below the melting temperature of DCPD (8-9 °C), and thus DCPD is a solid at this temperature. Presumably, the solid monomer is unable to dissolve catalyst from the modified rheometer plate, and therefore no reaction is expected to take place. Upon increasing the temperature to 13 °C and 28 °C, evolution of G' is observed, with gelation times of 1027 ± 48 and 364 ± 41 seconds, respectively. However, increasing the temperature to 40 °C initially appears to slow the polymerization, contrary to what may be expected at elevated temperatures. Visual observation of the rheometer plate immediately after this experiment revealed a layer of polymer film directly covering the modified bottom plate, on top of which was a layer of liquid monomer DCPD (Figure 3). It is believed that the inference that the polymerization kinetics decreases at higher temperatures is not correct, and instead the polymerization reaction is so rapid that it quickly gels around catalyst particles, quenching further dissolution of catalyst and slowing diffusion of catalyst into the upper layer of liquid. The fact that samples at elevated temperatures exhibits obvious property gradients through the sample thickness complicates any rheological interpretation of this data, but these results demonstrate that the self-healing mechanism is extremely sensitive to moderately high healing temperatures.
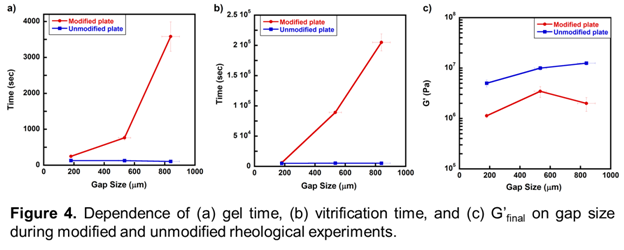
Figure 3 shows that the stiffest layers of polymer exists in close proximity to the bottom rheometer plate, and stiffness decreases further from the modified plate surface. The most likely reason for this property gradient is that catalyst dissolves into the bottom layers of the monomer and must diffuse towards the top layers. This led to the question of whether healing would be significantly affected based on the thickness of the liquid monomer sample, in which thicker samples may require more time to diffuse catalyst to the top layers of monomer. With respect to self-healing polymers, this question has implications towards the capability of healing cracks of different thickness. Hence, healing at gap sizes of 180 ± 9, 534 ± 41, 839 ± 58 μm were tested. Figure 4 shows how gelation time, vitrification time, and G'final change with changing gap size. Also, as a control test, Figure 4 shows the dependence of these three variables on gap size in unmodified rheology experiments, in which catalyst and monomer was premixed (2mg/ml catalyst/monomer) and injected on an unmodified bottom rheometer plate. It can be seen that at larger gap sizes, the speed of healing is indeed significantly affected.