www.acsprf.org
Reports: DNI148906-DNI1: Synthesis and Application of Functionalized Organocopper Species by C-Si Bond Activation of Organosilane Precursors
Zachary Ball, PhD , Rice University
Funding from the PRF Doctoral New Investigator program has enabled my lab to build a broad program centered on transition-metal catalysis. We proposed to develop efficient methods to activate stable synthetic intermediates, focusing on copper chemistry and silane activation. We have achieved significant progress in developing copper-catalyzed organosilane activation into a viable tool for synthetic chemists, and these achievements are outlined in this report. In addition, the DNI grant has served as a launching point for expansion of the fundamental goals of this work into new research areas. We have expanded our ideas for the activation of chemically inert reagents from CSi bonds to a new copper-catalyzed approach to CH bond functionalization as well, recently published in Org. Lett. In this work, we pursue CH halogenation by a radical, 1-electron pathway inspired by metal-catalyzed, atom-transfer reactions. In a broader sense, copper(I) fluoride catalysis requires a commitment to ligand design and efficient ligand synthesis. In other projects, we have developed the use of peptide ligand scaffolds that are readily synthesized and optimized by automated methods.
We focused on CSi bond activation because selective synthetic methods that are safe, cheap, and environmentally benign continue to be of importance in organic chemistry. Organosilane reagents are important in this regard because they are typically stable to a wide range of functional groups and reaction conditions, allowing selective chemical transformations in multifunctional molecules. In addition, organosilanes are generally inexpensive and produce innocuous and easily removed polysiloxane byproducts. However, the classical use of organosilanes as carbon nucleophiles for CC bond formation is generally limited to intramolecular processes or reactions with extremely reactive electrophiles. Our work is aimed the activation and transmetalation of organosilanes with transition-metal catalysts to produce reactive organometallic intermediates from stable silane precursors. In particular, we are interested in the development of stable copper(I) fluoride complexes for the transmetallation of organosilanes, affording a new, general, and functional-group tolerant access to organocopper species. A key insight to allow development of these processes has been the use of heterocyclic carbene ligands to stabilize copper(I) species, to avoid problems of metal fluoride instability, and to control reaction selectivity.
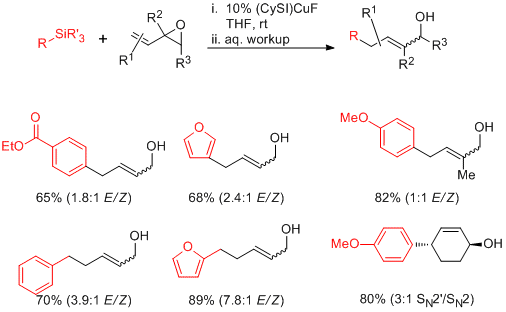
Following preliminary work from our labs which established the stoichiometric transmetalation of organosilane and copper complexes of the type LCuF (L = heterocyclic carbene), we moved toward the development of catalytic processes. Despite concerns about the ability to generate turnover in the absence of a stoichiometric fluoride activator, optimization of ligand structure resulted in the discovery that the complex (SICy)CuF is a competent catalyst for the coupling of organosilanes with vinylepoxide substrates at room temperature. Significant substitution and functional-group compatibility is tolerated, and the silane nucleophile can include (hetero)aryl and (hetero)benzylic groups.
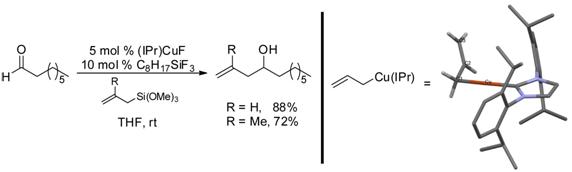
We have also examined the formation, structure and reactivity of allylcopper intermediates. Treatment of (IPr)CuF with an allylsilane results in the formation of the first synthesis of an allylcopper species, which could be characterized by NMR and crystallography. This process enabled the development of a simple protocol for catalytic aldehyde allylation. A catalytic amount of alkyltrifluorosilane was found to be essential for efficient catalysis. The role of this additive is under study; it appears to function primarily to facilitate catalyst turnover by allowing transmetalation from the presumed alkoxysilane product after 1,2 addition.
Funding from the PRF has also enabled us to broaden these concepts to CC bond-forming processes triggered by CSi activation generally. In work currently under review for publication, we demonstrated that copper-catalyzed silane activation enables intramolecular and intermolecular reactivity with a range of electrophiles, including 1,4-addition to enone and enoate substrates. In an especially exciting development, tandem cascades based on combinations of intra- and intermolecular CC bond-forming processes are possible. Silane substrates bearing a pendant alkyne undergo carbometalation to afford a new vinylmetal species which can be subsequently trapped by intermolecular electophiles such as enones, enoates, and vinyl epoxides. Intramolecular cases succeed for challenging cases such as 8-membered-ring formation, and recent results have demonstrated that control of absolute stereochemistry is possible through appropriate design of chiral ligands.
The processes developed with support from the PRF DNI grant provide new methodologies to synthetic chemists. Importantly, these studies provide reliable silane alternatives to toxic reagents such as organostannanes, and to sensitive or dangerous reagents such as organolithium and organomagnesium compounds. Finally, new catalytic methodologies with silanes provide new insight and opportunities for the synthesis of polysilanes and other organosilane materials.