www.acsprf.org
Reports: UNI149489-UNI1: Stereoselective Tin-Free Radical Reactions
Jake R. Zimmerman, PhD , Ohio Northern University
My research grouphas benefited tremendously from the support of the ACS-PRF-UNI grant that Ireceived. It has allowed me to fund four summer research students over the pastthree summers and purchase a variety of expendables that were needed for thisproject. One of the students is currently beginning their senior year at ONUand plans to attend graduate school in chemistry beginning next fall. The otherthree students are currently attending graduate school in chemistry. Because ofthis support, my group has accumulated a lot of exciting results and we wereable to publish two articles this year with two undergraduate co-authors. Anundergraduate research student and myself presented two posters at the spring2010 ACS National meeting in San Francisco, CA (abstract numbers ORGN 171 andORGN 161). I gave a presentation at the Pacifichem meeting in December 2010(abstract ID: 886) and had two undergraduate students present at the fall 2011ACS National meeting in Denver, CO (abstract numbers ORGN 594 and ORGN 596).Below is a summary of my group's progress over the past twelve months(September 1, 2010 through August 31, 2011).
Stereoselective free radical reactions have beenstudied over the past several decades; however, there are still severalobstacles to be overcome.[1]One major problem that plagues free radical processes is the use of organotinreagents.[2]These reagents are toxic and the byproducts can be very difficult to removefrom reaction mixtures. A variety of alternatives for organotin reagents havebeen presented in the literature,2 yet this is still a developingfield of research. Some research groups have also reported tin-free radicaladditions using Et3B[3]and Et2Zn[4] asinitiators and chain transfer reagents.
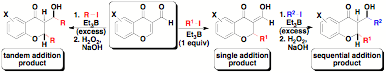
Another drawback tostereoselective free radical reactions is the lack of functional groupcomplexity in the products synthesized via these methods.2 Therefore,we set out to identify substrates that would produce useful, small moleculescaffolds utilizing tin-free radical processes. We ultimately focused on 3-formylchromone (1) as our radical acceptor. A variety of naturalproducts contain modified chromone frameworks that are related to thestructures produced in this new method.[5] Ultimately wewere able to demonstrate a highly diastereoselective tin-free tandem radicaladdition to 3-formylchromones yielding functionalized products with up to threecontiguous stereocenters (see Scheme 1 below).[6]
Scheme 1.Summary of tin-free radical additions to 3-formylchromones.
My group also worked on a green chemistry project thispast year. Over the past decade, a major focusof many research groups has been the development of environmentally benignreaction methodologies.[7]One key principle of green chemistry focuses on designing chemical processeswhich reduce or avoid the use of highly toxic substances.10 Manyfree radical processes require the use of alkyltin reagents since they areexcellent hydrogen atom sources and radical chain carriers.1 Twomajor problems with organtin reagents, however, is that they are toxic and thebyproducts can be very difficult to remove from reaction mixtures.2
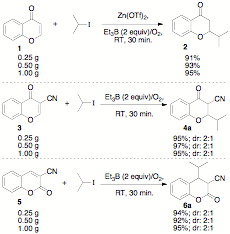
Another major principle of green chemistry is reducingor avoiding the use of solvents to prevent the generation of significantquantities of waste, decrease reaction times, reduce energy consumption, andlower batch sizes. Significant amounts of research has been carried out onsolvent-free or high concentration reaction methods, yet there are only aselect few reports on solvent-free radical reactions.[8]Therefore, we focused on a green chemistry free-radical project. We were ableto demonstrate a tin-free radical methodology using minimal solvents for thesynthesis of substituted chromones and coumarins (see Scheme 2 below).[9]
Scheme 2.Green chemistry: Tin-free radical reactions using chromones and coumarins up to1.00 g scale.
Insummary, my research group has studied tin-free radical reactions for the pastseveral years. We have made significant progress in this area and havepublished our work (with undergraduate coauthors) in peer-reviewed journals inthe field of organic chemistry. The ACS-PRF-UNI funding has really helpedpropel my research to the next level and I will continue these programs in thefuture.
([1]) (a) Sibi, M. P.; Manyem,S.; Zimmerman, J. Chem. Rev. 2003, 103, 3263-3295. (b) Sibi, M. P.; Rheault, T. R. In Radicalsin Organic Synthesis,Renaud, P., Sibi, M. P. Eds.; Wiley-VCH, Weinheim, 2001; pp 461-478. (c) Sibi,M. P.; Porter, N. A. Acc. Chem. Res. 1999, 32, 163-171.
([2]) For references onalternates to tin see: (a) Studer, A.; Amrein, S.; Schleth, F.; Schulte, T.;Walton, J. C. J.Am. Chem. Soc.2003,125,5726-5733. (b) Jang, D. O.; Cho, D. H. Synlett 2002, 1523-1525. (c) Khan,T. A.; Tripoli, R.; Crawford, J. J.; Martin, C. G.; Murphy, J. A. Org. Lett. 2003, 5, 2971-2974.
(3) (a)Brown, H. C.; Midland, M. M. Angew. Chem. Int. Ed. Engl. 1972, 11, 692-700. (b) Miura, K.; Ichinose, Y.; Nozaki, K.;Fugami, K.; Oshima, K.; Utimoto, K. Bull. Chem. Soc. Jpn. 1989, 62, 143–147.
(4) (a)Bertrand, M. P.; Coantic, S.; Feray, L.; Nouguier, R.; Perfetti, P. Tetrahedron, 56, 2000,3951-3961. (b) Bazin, S.; Feray, L.; Siri, D.; Naubron, J. -V.; Bertrand, M. P. Chem. Commun., 2002,2506-2507.
(5)(a) GŽrard, E. M. C.; BrŠse, S. Chem. Eur. J. 2008, 14, 8086-8089. (b) Overy, D.; Calati, K.; Kahn, J. N.;Hsu, M. J.; Martin, J.; Collado, J.; Roemer, T.; Harris, G.; Parish, C. A. Bioorg.Med. Chem. Lett. 2009, 19,1224–1227.
([7])(a)Anastas, P. T.; Kirchhoff, M. M. Acc. Chem Res. 2002, 35, 686; (b)Anastas, P. T.; Lankey, R., Green Chemistry: Theory and Practice; OxfordUniversity Press: New York, 1998; p 30.