www.acsprf.org
Reports: DNI950576-DNI9: Linking Ion Structure with Aromatic Solubility in Ionic Liquids
Wesley A. Henderson, PhD , North Carolina State University
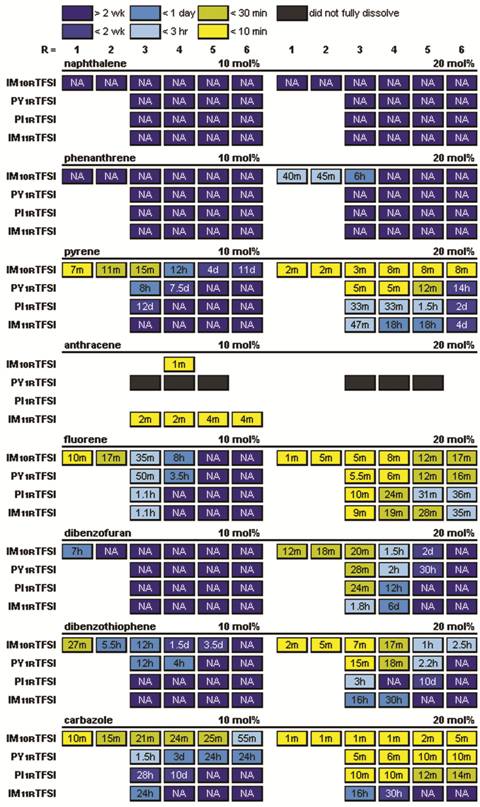
The solubility and recrystallization kinetics of polyaromatic compounds (PACs) in ionic liquids (ILs) has been explored initially for two classes of PACs with and without heteroatoms. The PACs were dissolved in the ILs and the crystallization rate was monitored. To complement this work, 1H-NMR was utilized to determine the PAC solubility in the ILs. Wide variations were observed for different PACs and IL cation structures. All of the ILs used in this study had the bis(trifluoromethanesulfonyl)imide (TFSI-) anion and one of the following four cations with varying alkyl chain lengths: 1-alkyl-3-methylimidazolium (IM10R+), 1-alkyl-2,3-methylimidazolium (IM11R+), N-alkyl-N-methylpyrrolidinium (PY1R+) or N-alkyl-N-methylpiperidinium (PI1R+) (Fig. 1). The solubility of the aromatic compounds (Fig. 2) increased significantly with increasing length of the IL cation alkyl substituent. Surprisingly, the aromatic solubility in the ILs increased in the following order: IM10R+ < PY1R+ < PI1R+ < IM11R+ (Table 1), which argues against the frequently cited supposition that pi-pi interactions are the primary controlling factor that determines the solubility of aromatic compounds in ILs.
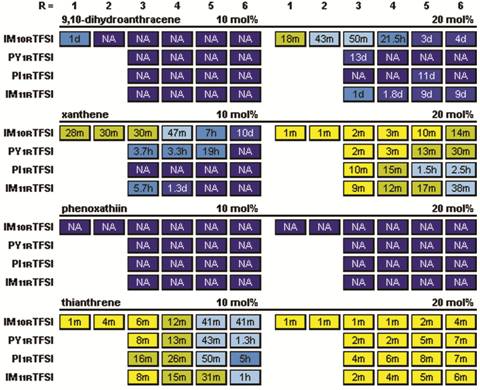
It was interesting to note that while anthracene had exceptionally low solubility in all of the ILs, phenanthrene and fluorene were both fairly soluble. The study was therefore extended to include related polycyclic (but not polyaromatic) compounds (Fig. 2 and Table 2). Xanthene and thianthrene were reasonably insoluble, whereas 9,10-dihydroanthracene and phenoxathiin both had high solubility in the ILs. The solubility trends can be roughly correlated with the variation in the compound melting temperature (Tm), although exceptions to this are found (i.e., pyrene). Also, the differences in solubility for the different classes on ILs were not as evident for these polycyclic materials and the order changed to: IM10R+ < PY1R+ ~ IM11R+ < PI1R+ (Table 2).
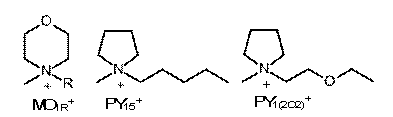
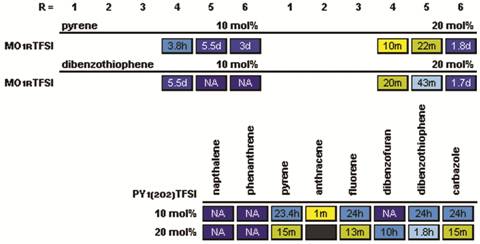
The study was also extended to examine the influence of heteroatoms (i.e., oxygen) on the IL cations. The N-alkyl-N-methylmorpholinium cations (MO1R+) are the same size and shape (essentially) as the PI1R+ cations, with one of the ring -CH2- segments replaced with an oxygen atom (Fig. 3). An oxygen atom was also added to the alkyl chains (i.e., PY1(2O2)+) for comparison with the PY15TFSI salt (Fig. 3). Interestingly, the oxygen atom on the ring (i.e., MO1RTFSI vs. PI1RTFSI salts) noticeably reduced the solubility of pyrene and dibenzothiophene, but the oxygen atom on the alkyl chain had a lesser (and perhaps negligible) affect on the solubility of these compounds. This initial study has resulting in clear correlations between ion structure and the solubility of aromatic materials in the ILs. These can be used to guide further studies which will provide insight into the molecular-level mechanisms for the dissolution of these materials in ILs.
Fig. 1. Ions in the ILs used for this study.
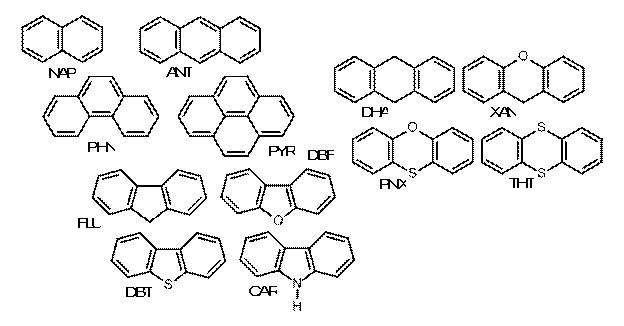
Fig. 2. PACs and polycyclic materials used for this study.
Table 1. Recrystallization times for PACs in the specified ILs with different alkyl chain lengths (after melting and then storing at room temperature).
Table 2. Recrystallization times for polycyclic compounds in the specified ILs with different alkyl chain lengths (after melting and then storing at room temperature).
Table 3. Melting temperature of the compounds studied.
Tm (°C)
naphthalene (NAP) 82
phenanthrene (PHN) 100
pyrene (PYR) 156
anthracene (ANT) 218
fluorene (FLU) 116
dibenzofuran (DBF) 82
dibenzothiophene (DBT) 98
carbazole (CAR) 245
9,10-dihydroanthracene 105
xanthene 102
phenoxathiin 54
thianthrene 153
Fig. 3. Ions in the ILs containing oxygen used for this study.
Table 4. Recrystallization times for polycyclic compounds in the specified ILs with different alkyl chain lengths (after melting and then storing at room temperature).