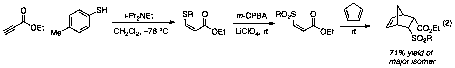www.acsprf.org
Reports: GB148322-GB1: Synthesis of Chiral Carboxylic Acid Derivatives via Three-Component Coupling Reactions with Ynoate Electrophiles
Chiles Wade Downey , University of Richmond
Introduction
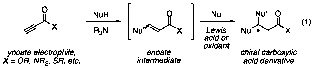
Our goal is to control the one-pot addition of two disparate nucleophiles to a single ynoate acceptor in order to yield a chiral carboxylic acid derivative (eq 1). Thus, simple ynoate electrophiles may be converted in one reaction vessel to densely functionalized, stereochemically complex cyclic or polycyclic systems. Successful development of this method represents a valuable advance for a number of reasons: 1) Ynoate esters are readily available from acetylene or propargyl alcohols. 2) Three-component coupling reactions are highly efficient. 3) These reactions rapidly construct complex synthons for target-oriented synthesis.
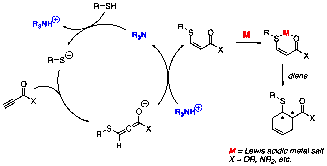
A proposed mechanism for a representative heteroconjugate addition-Diels–Alder process is outlined in Figure 1. First, a trialkylamine base (R3N) deprotonates a thiol nucleophile, generating a thiolate anion and a trialkylammonium cation. Conjugate addition by the thiolate provides an allenolate intermediate, which is protonated by the trialkylammonium salt to yield an enoate ester. The enoate then acts as a conjugate acceptor; in this example, it is activated by a Lewis acid (M) and undergoes Diels–Alder cycloaddition. Our ultimate goal is to control the formation of the new stereocenters by the use of a chiral auxiliary or a chiral metal catalyst.
Figure 1. Three-Component Coupling Reaction of Ynoates
Current Results
We planned a three-pronged approach toward the development of this three-component coupling reaction: 1) establishment of the amine-catalyzed conjugate addition of various nucleophiles to ynoates, 2) use of the conjugate addition products as dienophiles in Diels–Alder cycloadditions, and 3) development of an asymmetric variant. We now report that we have achieved the first two goals and are completing our study of the reaction scope for the racemic process.
1. Amine-Catalyzed Conjugate Addition to Ynoates
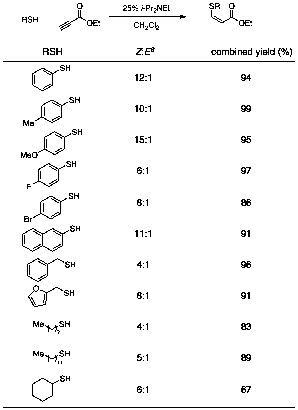
Several classes of nucleophiles undergo amine-catalyzed conjugate addition to ethyl propiolate, as described in Table 1. Nitroalkanes and phenols are promising, but our current work focuses upon thiol nucleophiles. Table 1 exhibits some representative examples: thiophenol and its derivatives require only catalytic amounts of amine and afford excellent yields with high Z:E ratios. Selectivity for aliphatic thiols suffers somewhat (e.g., entry x) but remains synthetically useful.
Table 1. Amine-Catalyzed Thioconjugate Addition to Ethyl Propiolate
aDetermined by 1H NMR spectroscopy of the unpurified reaction mixture
2. Thioether Oxidations
Our thioether adducts themselves are not active in Diels–Alder reactions under typical thermal or Lewis acidic conditions. Accordingly, we developed a one-pot two-step thioconjugate addition-oxidation sequence to produce a sulfone product under conditions that would also be amenable to Diels–Alder cycloaddition. Meta-chloroperbenzoic acid (m-CPBA) proved to be a convenient oxidant for our system. We found that 3 equiv m-CPBA produced the sulfone when reacted with thioether formed in situ. Close inspection of the 1H NMR spectrum of the unpurified reaction mixture revealed a large number of unidentified byproducts.
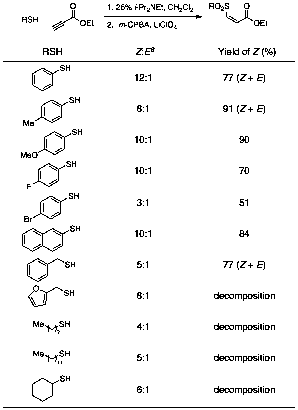
Because oxidation of the purified thioether proceeded in high yield with no byproduct formation, we sought to mitigate the effect any residual amine catalyst might have on the oxidation. We chose LiClO4 to act as a buffer because of its ability to catalyze the Diels–Alder reaction we envisioned as the third step of our one-pot process. This one-pot conjugate addition-oxidation sequence was quite effective for thiophenol derivatives and for benzyl mercaptan, but reaction mixtures generated with other aliphatic thiols (cyclohexanethiol, octanethiol, dodecanethiol) were complex under one-pot conditions (Table 2). Oxidation of the purified thioethers, however, was consistently clean, so our current focus is modification of our experimental procedure to better accommodate aliphatic thiols.
Table 2. One-Pot Thioconjugate Addition-Oxidation Reaction
aDetermined by 1H NMR spectroscopy of the unpurified reaction mixture
3. Diels–Alder Cycloadditions
The Diels–Alder portion of our reaction was first optimized using the purified sulfone oxidation product. A survey of Lewis acid catalysts at various temperatures showed LiClO4 (0.5 equiv) to be the optimal catalyst for cycloaddition by cyclopentadiene, providing the product in high conversion and endo:exo ratio (Table 3). Attempts to expand the reaction scope beyond cyclopentadiene have been unsuccessful to date. Cyclohexadiene reacts with very high diastereoselectivity, but yield is inconsistent and does not reproducibly exceed 40%. Other dienes tested have been wholly unreactive.
Nonetheless, addition of LiClO4 to our procedure for the one-pot three-step thioconjugate addition-oxidation-Diels–Alder sequence generated impressive leads. By adding the LiClO4 immediately prior to the oxidation step, then continuing under our normal conditions, we were able to achieve a 71% yield of the major isomer for the cyclopentadiene reaction when 4-toluenethiol was employed as the initial nucleophile (eq 2). This yield is a substantial increase over the 15% yield observed when no additive is present during the oxidation step, and provided the toehold that allowed us to begin expansion of the reaction scope.
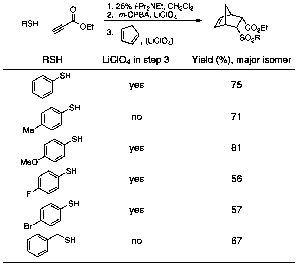
The same reaction conditions employed in eq 2 were successful for benzyl mercaptan as well, although a higher catalyst loading of the Lewis acid (1.0 equiv) was necessary to achieve reasonable yield (67%). Surprisingly, extension of these reaction conditions to other aromatic and aliphatic thiols generally failed to achieve high diastereoselectivity in the cycloaddition step. After extensive experimentation during the summer of 2011, we have discovered that addition of 1.0 equiv LiClO4 during both the oxidation step and the cycloaddition provides remarkably consistent results for a range of substituted thiophenol derivatives (Table 3).
Table 3. One-Pot Heteroconjugate Addition-Oxidation-Diels–Alder Reactions
Conclusion
We have established an effective, high-yielding method for the three-component coupling of ethyl propiolate, aryl thiols, and cyclopentadiene. We will complete our work with aryl thiols by the end of the calendar year, and should be able finish our work with aliphatic thiols in Spring 2012. We anticipate submission of our preliminary work in this program to an important journal by early summer. Our other long-term goals include the development of asymmetric versions of our reaction.